Definition
Limit test is defined as a quantitative or semi-quantitative test designed to identify and control small quantities of impurities which are likely to be present in the substance.
Principle
It is based upon the chemical reaction between silver nitrate and soluble chloride in the presence of dilute nitric acid to give opalescence of silver chloride. The opalescence produced is compared with the standard solution. If the opalescence in the sample is less than the standard, it passes the test. If it is more than the standard, it fails the test.
Apparatus Required
Nessler cylinders
Glass rod
Stand
Chemicals Required
Dilute nitric acid (10%)
Silver nitrate (5%)
Sodium chloride
Reaction

Procedure
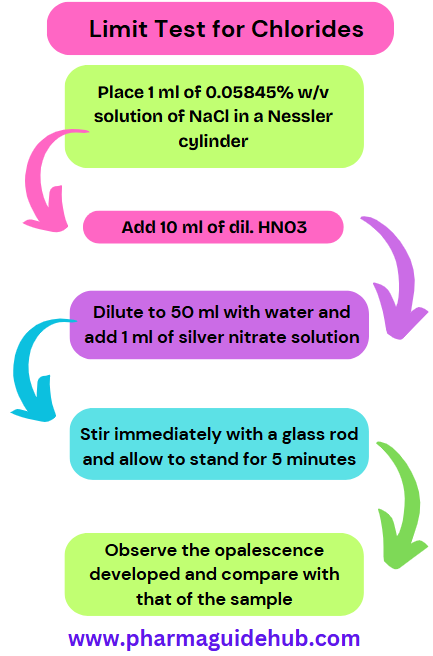
Take two 50 ml Nessler cylinders. Label one as “Test” and the other as “Standard”.
| Standard | Test |
| 1. Place 1 ml of 0.05845% w/v solution of NaCl in a Nessler cylinder. | 1. Dissolve the specified quantity of the substance in distilled water and transfer to Nessler cylinder. |
| 2. Add 10 ml of dil. HNO3. | 2. Add 10 ml of dil. HNO3. |
| 3. Dilute to 50 ml with water and add 1 ml of silver nitrate solution. | 3. Dilute to 50 ml with water and add 1 ml of silver nitrate solution. |
| 4. Stir immediately with a glass rod and allow to stand for 5 minutes. | 4. Stir immediately with a glass rod and allow to stand for 5 minutes. |
| 5. Observe the opalescence developed and compare with that of the sample. | 5. Observe the opalescence developed and compare with that of the standard. |
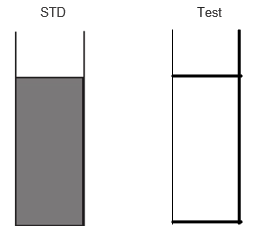
The opalescence in STD is seen more than that of the Test; thus the sample passes the limit test of chloride