Limit Tests
Limit = A value or amount that is likely to be present in a substance. Test = To examine or to investigate
Impurity = A foreign matter present in a compound
Definition
Limit test is defined as a quantitative or semi-quantitative test designed to identify and control small quantities of impurities which are likely to be present in the substance.
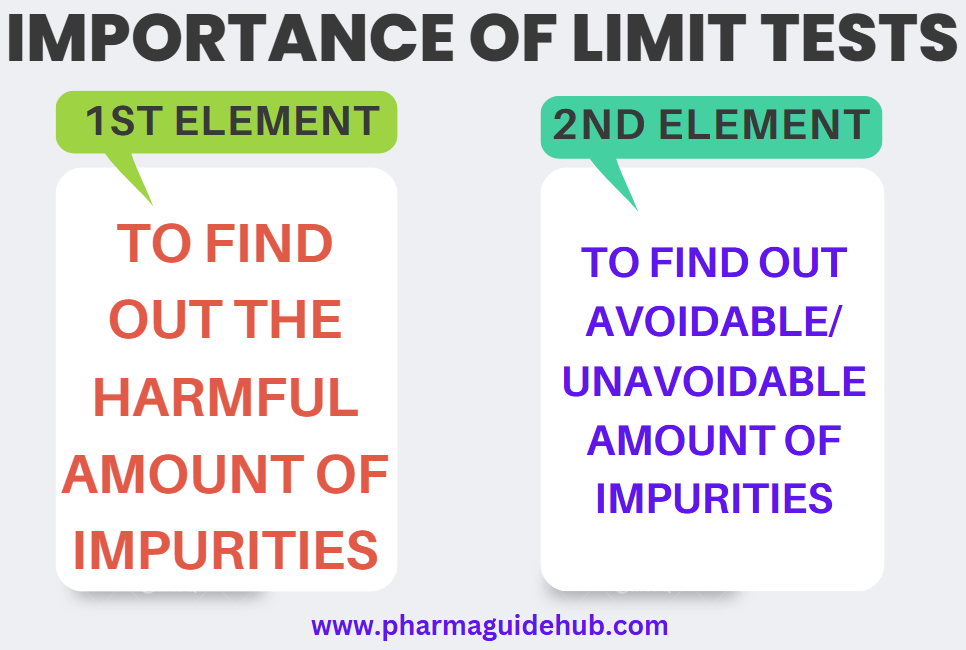
Importance of Limit Tests
To find out the harmful amount of impurities
To find out avoidable/ unavoidable amount of impurities.
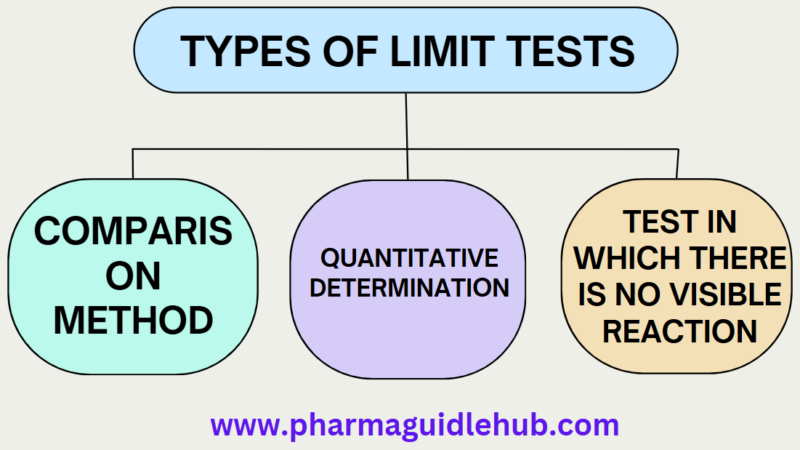
Types of Limit Tests
Comparison method
Quantitative determination
Test in which there is no visible reaction
General principle
If the sample is lighter (in colour/turbidity/opalescence) than the standard solution then it is within the pharmacopoeia! limit (accepted).
If the sample is darker/heavier than the standard solution then it is above the pharmacopoeia! limit (rejected).
Specificity of a Limit Test : A given limit test for a trace impurity should involve some selective reaction of the reagent with the trace impurity under consideration/ detection specifically characteristic only to it.
Sensitivity of a Limit Test : As most of the limit tests involve dilute solutions and results are based on concentration of the trace impurity, the results may take longer duration to become observable or appreciable. Thus, consideration of duration of test need to be of prime consideration in designing the limit test.
NESSLER Cylinder (IP appendix VII A127)
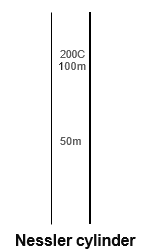
It is a clear glass cylinder with normal capacity of 50 ml, the overall height is about 15 cm, the external height to be 50 ml marked 11.0 to 12.4 cm, the thickness of the wall is around 1.0 to 1.5 mm and the thickness of the base is about 1.0 to 3.0 mm. The external height to the 50 mark of cylinders used for the test must not differ by more than 1 mm.
General Precautions:
- The liquid used must be clean and filtered if necessary.
- The Nessler cylinder must be made of colourless glass and of the same inner diameter.
- Detecting opalescence or colour development must be performed in daylight.
- Comparison of turbidity, it should be done against a black background.
- Comparison of colour, it should be done against a white background.