OBJECTIVE:
To lay down the procedure for manual inspection of tablets and capsules.
SCOPE:
This SOP is applicable to the procedure for manual inspection of tablets and capsules.
RESPONSIBILITY:
Initiator Officer/Designee: Production shall perform the operation activity as per SOP.
Initiator Executive/Designee: Production shall ensure the compliance of the SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
PROCEDURE:
Inspection of tablets and capsules is a critical step in pharmaceutical manufacturing to ensure product quality and patient safety. The process involves visually examining the tablets and capsules for any defects or inconsistencies, such as discoloration, cracks, chips, or foreign particles. The inspection can be done manually by trained personnel or automatically using sophisticated vision systems. The inspection process also includes verifying the identity, strength, purity, and other characteristics of the tablets and capsules. Any defective or suspect products are removed from the batch and disposed of according to established procedures. The inspection of tablets and capsules is a regulatory requirement in many countries and is essential for maintaining the safety and efficacy of pharmaceutical products.
Check the cleanliness of area and equipment in inspection area. Ensure cleanliness of area and equipment done as per SOP.
Ensure line clearance from IPQA before starting the activity as per line clearance procedure.
Transfer the tablets/capsules for inspection after verification of labels and cross check the weight of container.
Before starting of batch get HDPE containers/crates to the area as per SOP.
Place HDPE containers/crates with double lined polybag on SS stool below each outlet of SS inspection table to collect inspected tablets.
Place poly bags on surface of SS inspection table, without blocking the good tablet outlet chute.
Pour tablets/capsules to be inspected in the center of SS inspection table in such a way that all inspection personnel have enough space to inspect the tablets/capsules.
Take one bowl full of tablets/capsules and inspect with continuous tilting by hand so that all side of tablets/capsules are visualized by inspection personnel. Ensure that there are no further defects before passing the tablets/capsules to the outlet chute.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/manual-inspection-of-tablets-and-capsules/
The rejected tablets/capsules shall be kept in red colour bowl along with “REJECT LABEL”. Finally collect the rejected tablets from all red colour bowls to red colour HDPE container along with “REJECT LABEL”. After completion of inspection send rejected tablets for disposal as per SOP “Disposal Procedure for Rejections during manufacturing and Packing”.
Inspected tablets shall be kept in pre weighed HDPE containers/crates with double lined polybag and “PRODUCT LABEL”
- Ensure that qualified inspection personnel only do the inspection activity. Qualification of the personnel involved in the inspection activity shall be carried out against the predefined protocol.
- Personnel qualification for the tablet/capsule inspection shall be displayed in the inspection area as per the format.
- After every one hour personnel performing the inspection activity should take rest for five minute or another person should replace him.
The concern inspection personnel shall sign on product label. Check weight of the container after inspection and record the weighing details in BMR.
Enter the details of manual inspection and cleaning in “Equipment Log Book”. Enter the area used for the inspection in the Remark column of the Log Book.
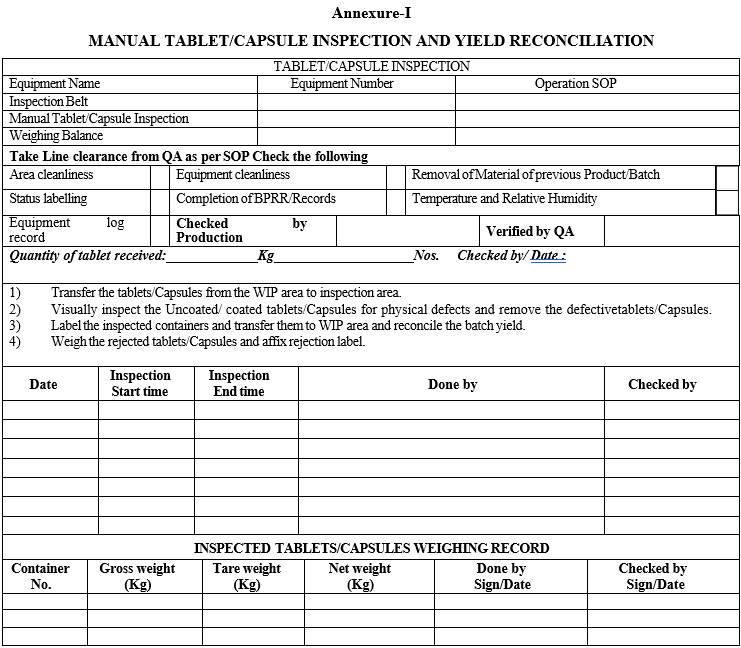
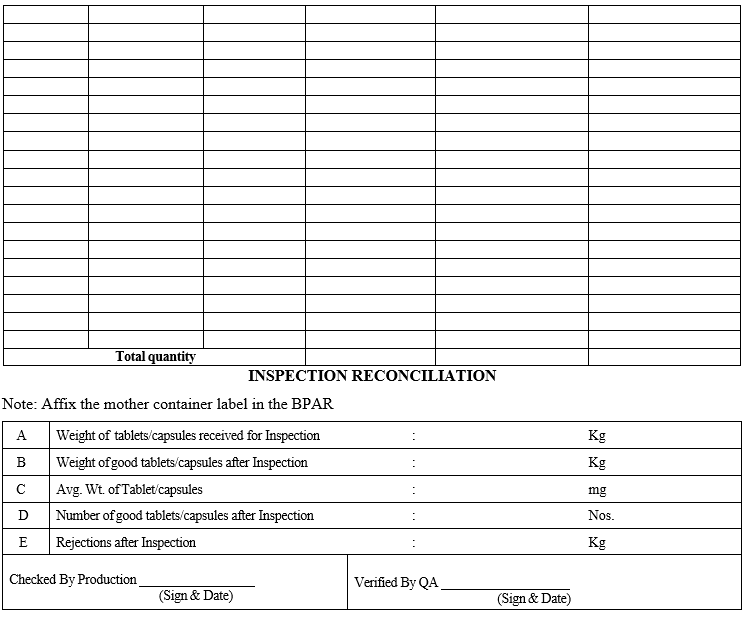
Manual Tablets/Capsules inspection and yield reconciliation details shall be recorded in Format-1, MANUAL TABLET/CAPSULE INSPECTION AND YIELD RECONCILIATION.
Cleaning:
Type “A” cleaning of inspection room, SS inspection Table, scoops, red colour bowls and chair.
- Ensure that the status label shall be updated as “TO BE CLEANED”.
- Ensure that all containers and tablets of previous batch removed from the area.
- Remove the poly bags from surface of SS inspection table and discard in waste bin.
- Clean the inner and outer surface of above-mentioned equipment with fresh dry lint free cloth.
- Ensure the cleanliness of the room waste bin.
- Enter the details of cleaning in the equipment log book as per the Annexure of SOP
- Frequency: After every batch of the same product.
Type “B” cleaning of inspection room, SS inspection Table, scoops, red colour bowls and chair.
- Ensure that the status label shall be updated as “TO BE CLEANED”.
- Ensure that all containers and tablets of previous batch removed from the area.
- Remove the poly bags from surface of SS inspection table and discard in waste bin.
- Place the Scoops and red colour bowls in poly bags and transfer same from inspection to the washing area.
- Clean the scoops and accessories as per SOP.
- Place the cleaned scoops and red colour bowl in polybag/shrink film and update the status label as “CLEANED” label.
- Clean the SS inspection table and chair with lint free cloths dipped in potable water and followed by purified water and finally wipe with 70% IPA.
- Clean the area as per the SOP.
- Enter the details of cleaning in the equipment logbook as per the Annexure of SOP
- Frequency: After every batch of the different product.
REFERENCES:
Not Applicable
ANNEXURES:
| Annexure Number | Title of annexure |
| Annexure-I | MANUAL TABLET/CAPSULE INSPECTION AND YIELD RECONCILIATION |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
Master Copy : Quality Assurance Department
Controlled Copy No. 01 : Head Quality Assurance
Controlled Copy No. 02 : Head Production
ABBREVIATIONS:
| PD | : | Production |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| BMR | : | Batch manufacturing record |
| HDPE | : | High density polyethylene |
| IPQA | : | In process Quality Assurance |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
MANUAL TABLET/CAPSULE INSPECTION AND YIELD RECONCILIATION
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/manual-inspection-of-tablets-and-capsules/




