- OBJECTIVE:
To lay down a procedure for Material Codification In Pharmaceutical.
- SCOPE:
This SOP is applicable for Material Codification In Pharmaceutical at {Company Name} {Company Location}.
- RESPONSIBILITY:
QA, QC, Production, F&D Warehouse Officer /Executive & Purchase – Follow the instruction as per procedure.
QA Sr. Executive – Review and technical correction of SOP.
QA Head –Training, approval & implementation of SOP.
- ACCOUNTABILITY:
Head Quality Assurance
- PROCEDURE:
- The materials shall be divided into Four types depending on the characteristic of the material,These types are as under:
- Raw Material: This includes all APIs & Excipients. The description of the material shall be as per the pharmacopoeia & those which are non-pharmacopoeial shall be described as per the trade name/In-house/generic name which is commonly used.
- Packaging Material: This includes all types of packaging materials used in the packing. For Example:-
Carton, BOPP Tape, Shipper. CRC Cap, & Labels.
- Finished Products: This includes final finished product.
- Miscellaneous Item: This includes miscellaneous item usedapart from product manufacturing,
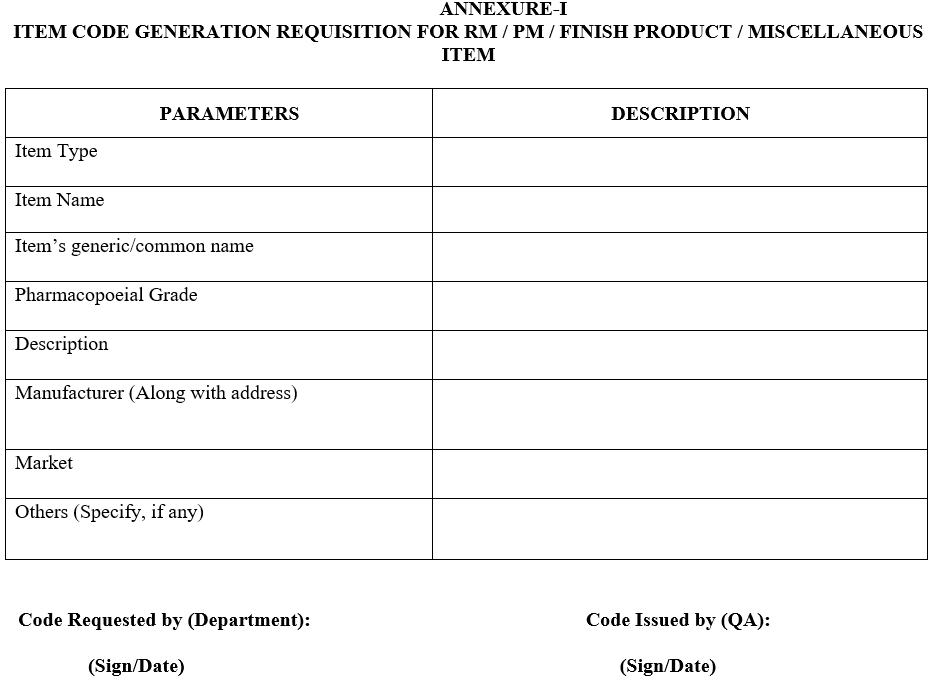
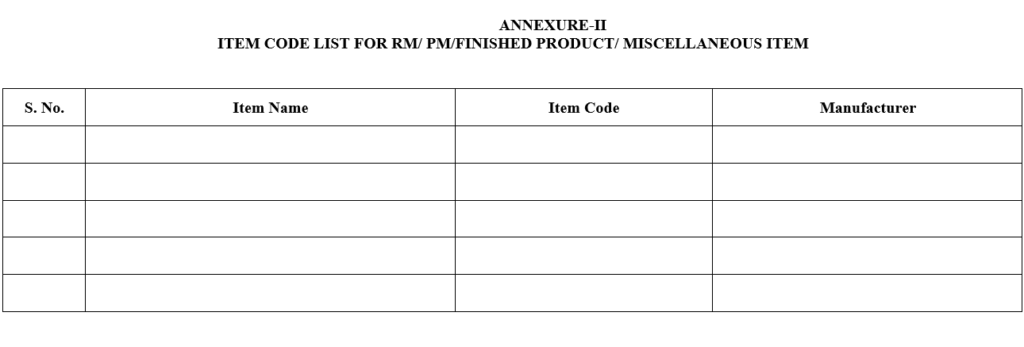
- After receiving the request from concerned department as per Annexure-I, QA will issue the item code number to Raw material, Packing Material & Finished Products respectively and prepare a list of the item code as per Annexure-II.
- Material Code:
- The Material code for Raw Material, Packing Material & Finished Product shall be assigned a number with seven alphanumeric characters.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/material-codification-in-pharmaceutical/
Material Code: RM/0001, PM/0001, FP/0001
Where,
The 1st two Characters indicate the material types.
For Example:-
For Raw material, the code shall be RM.
For Packaging material, the code shall be PM.
For Finished Product, the code shall be FP.
3rd Character is a Separator.
0001 indicates sequential Number.
- Material Code in the case of Miscellaneous Item shall be Assigned as M/0001
Where, M- Code for miscellaneous Items
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
| Annexure-I | Item code generation requisition for RM / PM / Finish Product / Miscellaneous Item |
| Annexure-II | Item code list for RM/ PM/Finished Product/ Miscellaneous Item |
- DISTRIBUTION:
| Control copy No. 1 | : | Head Quality Assurance |
| Control copy No. 2 | : | Head Quality Control |
| Control copy No. 3 | : | Head Production |
| Control copy No. 4 | : | Head Purchase |
| Control Copy No.5 | Head Warehouse | |
| Master Copy | : | Quality Assurance Department |
- ABBREVIATIONS:
| QA | : | Quality Assurance |
| SOP | : | Standard Operating Procedure |
| No. | : | Number |
| APIs | : | Active Pharmaceutical Ingradients |
| BOPP | : | Biaxially Oriented Polypropylene |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/material-codification-in-pharmaceutical/
ANNEXURE-I
ITEM CODE GENERATION REQUISITION FOR RM / PM / FINISH PRODUCT / MISCELLANEOUS ITEM
ANNEXURE-II
ITEM CODE LIST FOR RM/ PM/FINISHED PRODUCT/ MISCELLANEOUS ITEM
Frequently Asked Questions?
Q: What is material codification in the pharmaceutical industry?
A: Material codification involves assigning unique codes to various pharmaceutical components for systematic identification and tracking purposes.
Q: Why is material codification important?
A: Material codification enhances inventory management, streamlines procurement, and ensures accurate tracking of raw materials, aiding in quality control and regulatory compliance.
Q: How is material codification implemented?
A: Pharmaceutical companies typically utilize standardized coding systems, such as barcodes or alphanumeric codes, to assign unique identifiers to each material, facilitating efficient inventory control and traceability.
Q: What benefits does material codification offer?
A: Material codification improves inventory accuracy, reduces errors, accelerates order processing, and enhances overall supply chain efficiency in the pharmaceutical sector.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/material-codification-in-pharmaceutical/




