- OBJECTIVE:
To lay down a procedure for Material Entry & Exit at Security Gate.
- SCOPE:
This SOP is applicable for Material Entry & Exit at Security Gate at {Company Name} {Company Location}.
- RESPONSIBILITY:
- Security Supervisor shall be responsible for follow the instruction as per procedure.
- HR Manager/Designee shall be responsible for review, technical correction, Training & monitoring of SOP.
- QA Manager shall be responsible for approval of SOP.
- ACCOUNTABILITY:
QA Manager and HR Manager shall be accountable for the implementation and compliance of SOP.
- PROCEDURE:
- Returnable & non returnable register shall be filled in event of material dispatched from the factory.
- Whenever material is received, proper entry shall be made in the inward register and inform the concerned department immediately.
- Whenever material is dispatched from the factory premises, entry shall be made on outward register.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/material-entry-exit-at-security-gate/
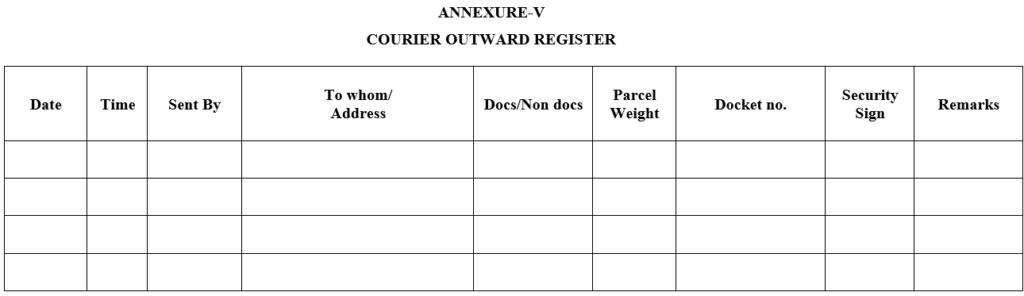
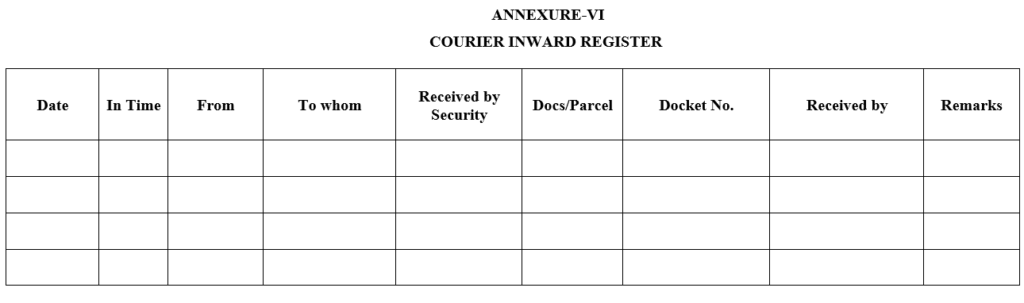
- Whenever courier received proper entry shall be made in courier inward register and handover the concern person takes the signature in respective register.
- Whenever courier dispatched proper entry shall be made in respective register.
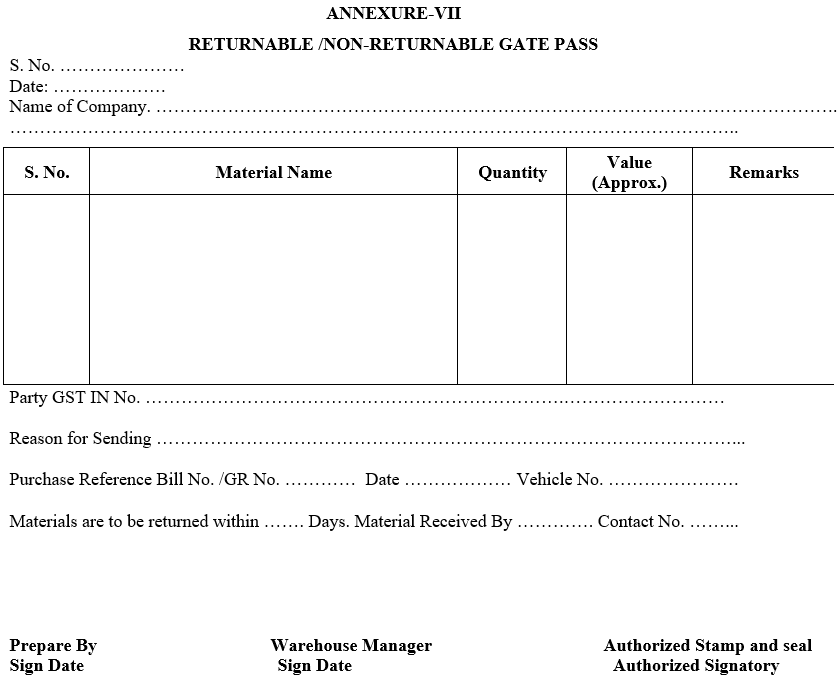
- Returnable or non returnable gate pass shall be made in the event of any material dispatched from the factory premises.
- The security person shall ensure that gate pass is dully filled & signed by concerned authorized persons whenever material dispatched from the factory premises.
- REFERENCES:
Not Applicable.
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
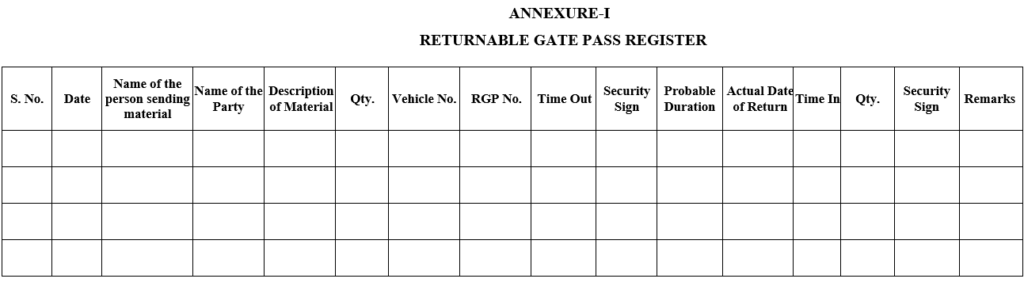
| Annexure-I | Returnable Gate pass register |
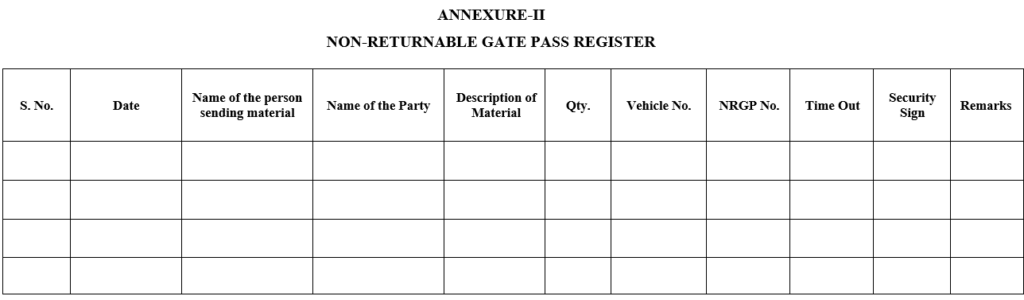
| Annexure-II | Non-returnable Gate pass register |
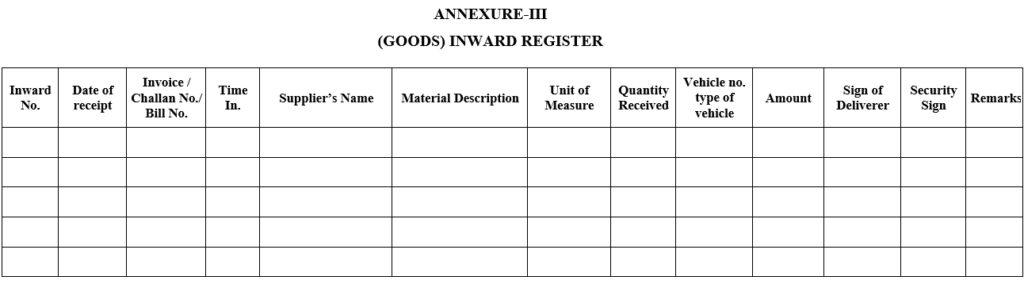
| Annexure-III | Goods inward register |
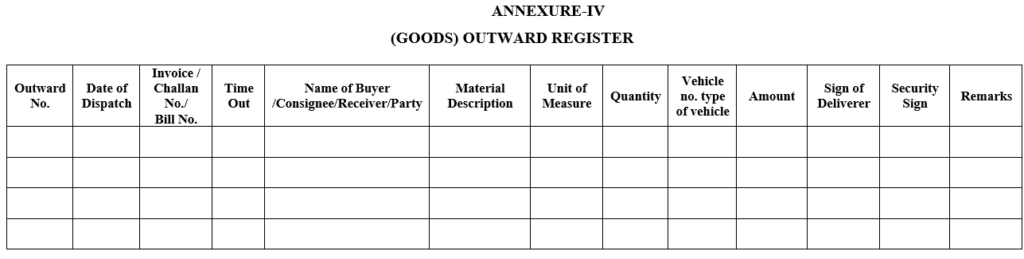
| Annexure-IV | Goods outward register |
| Annexure-V | Courier outward register |
| Annexure-VI | Courier inward register |
| Annexure-VII | Returnable / non-returnable gate pass |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
| Controlled Copy No. 01 | : | Manager Quality Assurance |
| Controlled Copy No. 02 | : | Manager HR |
| Master Copy | : | Quality Assurance Department |
- ABBREVIATIONS:
| No. | : | Number |
| Dept. | : | Department |
| Ltd. | : | Limited |
| QA | : | Quality Assurance |
| RGP | : | Returnable Gate Pass |
| NRGP | : | Non-Returnable Gate Pass |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not applicable | To be written manual |
ANNEXURE-I
RETURNABLE GATE PASS REGISTER
ANNEXURE-II
NON-RETURNABLE GATE PASS REGISTER
ANNEXURE-III
(GOODS) INWARD REGISTER
ANNEXURE-IV
(GOODS) OUTWARD REGISTER
ANNEXURE-V
COURIER OUTWARD REGISTER
ANNEXURE-VI
COURIER INWARD REGISTER
ANNEXURE-VII
RETURNABLE /NON-RETURNABLE GATE PASS
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/material-entry-exit-at-security-gate/
Frequently Asked Question?
Q: What document is used to track materials leaving the factory?
A: Both a returnable/non-returnable register and a gate pass are used to track materials leaving the factory.
Q: When is a returnable register used?
A: A returnable register is used for materials expected to return to the factory, like packaging containers.
Q: When is a non-returnable register used?
A: A non-returnable register is used for materials that will not be returned, like finished products being shipped to customers.
Q: What information is recorded in the inward and outward registers?
A: The inward and outward registers should record details like material description, quantity, date, supplier/customer information, and department involved.
Q: How is the receiving department notified when materials arrive?
A: The receiving department should be informed immediately by security person upon material arrival with an entry made in the inward register.
Q: How are couriers tracked?
A: Separate inward and outward registers are used to track incoming and outgoing couriers. The recipient should sign the register upon receiving a courier.
Q: What information is included on a gate pass?
A: The gate pass should include details like material description, quantity, recipient information, vehicle details, and authorization signatures.
Q: Who is responsible for ensuring the gate pass is properly completed?
A: The security personnel are responsible for verifying that the gate pass is filled out and signed by authorized personnel before allowing material to leave the premises.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/material-entry-exit-at-security-gate/


Thank you, I have just been looking for information about this topic for ages and yours is
the best I’ve came upon so far. But, what about the conclusion?
Are you sure concerning the source?
It’s in reality a great and useful piece of information. I am glad that you shared this useful information with us.
Please stay us up to date like this. Thanks for sharing.
Wow, superb blog layout! How long have you been blogging for?
you made blogging look easy. The overall look of your site is wonderful, let alone the content!