OBJECTIVE:
To lay down the procedure for carrying out microbiological examination of non sterile substances and products on reduced quantity of sample.
SCOPE:
This SOP is applicable for the procedure for carrying out microbiological examination of non sterile substances and products on reduced quantity of sample at {Company Name} Location}.
RESPONSIBILITY:
Microbiologist: is responsible to perform the activity as per SOP.
In charge- Microbiology- is responsible to ensure compliance as per SOP.
Head/Designee Quality Control – Shall be responsible for ensuring compliance as per SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
PROCEDURE:
Carryout the determination under conditions designed to avoid accidental contamination of the sample to be examined. The precaution taken to avoid contamination must be that they do not affect any microorganisms that would be revealed.
If the product to be examined has antimicrobial activity this must be adequately neutralized. If neutralizer is used for this purpose their efficacy and non toxicity to microorganisms shall be demonstrated.
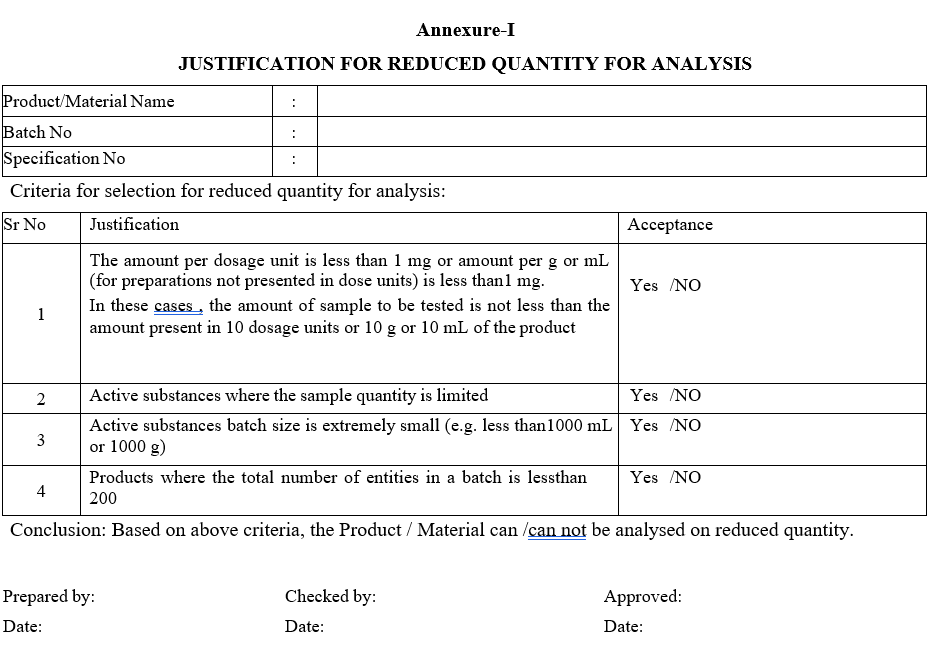
For each substances selected to be analysed by using reduced sample quantity, tabulate the justification based on the criteria listed under scope as per Format-I.
In case the sample quantity tested is less than the compendia recommendation, then the results shall be represented accordingly as per the quantity tested. The sample quantity shall be selected based on the criteria mentioned in the scope.
For example the sample quantity to be selected as per criteria. The proposed batch size for xxx is 100g and the reduced sample quantity for testing will be 1% of 100 g i.e. 1g. Accordingly the reduced sample quantity is as follows:
MLT method validation:
a) Microbial Enumeration Test method validation: 5 Microorganisms x 100 mg = 500 mg
b) Test for Specified Microorganisms method validation: 7 Microorganisms x 100 mg = 700mg. Total quantity for MLT method validation (a+b) : 1.2 g.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/microbiological-examination-of-non-sterile-substances-and-products-on-reduced-quantity-of-sample/
Sample quantity for routine testing:
a) Microbial Enumeration Test: 200 mg
b) Test for Specified Microorganisms method validation: 700 mg. Total quantity for MLT routine testing(a+b): Around 1g.
Preferably 1:10 dilution shall be considered for analysis. For example, 1.4 g or 1.4 mL of sample to be dissolved in 12.6 mL of diluent. If 1:50 or 1:100 dilution to be used, diluent volume shall be considered accordingly.
Note: If sample quantity is less or more than 1.4 g the diluents quantity shall be decreased or increased accordingly based on dilution.
Pour plate method:
Transfer the selected quantity of sample in the suitable volume of suitable diluent. Sample dilution, pretreatment e.g Heat treatment, pH adjustment etc should be carried as per recommendation provided in the method suitability. For 1:10 dilution , the total volume shall be made up to 14 mL (solution A).
Pipette out 1 mL in duplicate for total aerobic microbial count and total yeast and mold count. Carry out the further testing for media pouring, incubation, observation and result interpretation refer GTP.
For test for specified microorganisms, incubate the remaining quantity (equivalent 1 g or mL of sample i.e for a 1:10 dilution, 10 mL) of sample solution (solution A) at 30- 35°C for 18-24 hours and carry out further testing as per GTP.
Membrane filtration method:
Transfer the selected quantity of sample in the suitable volume of suitable diluent. Sample dilution, pretreatment e.g Heat treatment, pH adjustment etc. should be carried out as per recommendation provided in the method suitability. For 1:10 dilution , the total volume shall be made up to 14 mL (solution A).
Filter 1 mL each for total aerobic microbial count and total yeast and mold count. Carry out the further testing for media pouring, incubation, observation and result interpretation refer GTP.
For test for specified microorganisms, filter the sample solution (solution A) equivalent 1 g or mL of sample through membrane filter. Wash the filter as per method suitability. Transfer the filter to 100 mL soyabean casin digest medium and incubate at 30-35°C for 18-24 hours and carry out further testing as per GTP.
Note: If sample quantity is less or more than 1.4 g the diluents quantity shall be decreased or increased accordingly based on dilution.
For test for specified microorganisms:
Test for Bile Tolerant Gram Negative Bacteria: Transfer 1 mL of enriched solution A to 100 mL of Enterobacteria Enrichment Broth Mossel and incubate at 30-35°C for 24-48 hours and carry out further testing as per GTP.
Test for Escherichia coli: Transfer 1 mL of enriched solution A to 100 mL of MacConkey Broth and incubate at 42-44°C for 24-48 hours and carry out further testing as per GTP.
Test for salmonella: Transfer 0.1 mL of enriched solution A to 10 mL of Rappaport vassiliadis salmonella enrichment broth and incubate at 30-35°C for 18-24 hours and carry out further testing as per GTPs.
Test for Pseudomonas aeruginosa: Streak a loop full from enriched solution A on Cetrimide agar and carry out further testing as per GTP.
Test for Staphylococcus aureus: Streak a loop full from enriched solution A on Mannitol salt agar and carry out further testing as per GTP.
Test for Candida albicans: Transfer 1 mL of enriched solution A to 100 mL of Sabouraud dextrose Broth and incubate at 30-35°C for 3-5 days and carry out further testing as per GTP.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/microbiological-examination-of-non-sterile-substances-and-products-on-reduced-quantity-of-sample/
REFERENCES:
United States Pharmacopoeia
ANNEXURES:
| Annexure No. | Title of annexure |
| Annexure-I | Justification for reduced quantity for analysis |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control (Micro.)
- Master Copy : Quality Assurance Department
ABBREVIATIONS:
| No. | : | Number |
| GTP | : | General Testing Procedure |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
JUSTIFICATION FOR REDUCED QUANTITY FOR ANALYSIS
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/microbiological-examination-of-non-sterile-substances-and-products-on-reduced-quantity-of-sample/