OBJECTIVE:
To lay down a procedure for operation of Anemometer.
SCOPE:
This SOP is applicable for operation of Anemometer at {Company Name} {Company Location}.
RESPONSIBILITY:
Technical Assistant – To ensure the Calibration and operation of Anemometer.
Head – Engineering shall ensure compliance as per SOP.
ACCOUNTABILITY:
Engineering Head
Head Quality Assurance
ABOUT ANEMOMETER:
An anemometer is a device used to measure wind speed or air velocity. In the pharmaceutical industry, it plays a crucial role in ensuring the quality and safety of products. Here’s how:
Airflow Monitoring:
- Cleanroom Validation: Anemometers are used to verify the airflow patterns and velocities within cleanrooms, ensuring they meet stringent standards to prevent contamination.
- HVAC Systems: They help monitor the efficiency and proper functioning of HVAC systems, which control temperature, humidity, and airflow within manufacturing areas.
Product Testing:
- Spray Dryers: Anemometers measure the air velocity within spray dryers, influencing the drying rate and particle size distribution of powdered products.
- Fluid Bed Dryers: They assess the airflow patterns and velocities within fluid bed dryers, optimizing the drying process and product quality.
Environmental Monitoring:
Outdoor Air Quality: Anemometers help monitor outdoor wind speed and direction, which can impact the quality of incoming air and potential airborne contaminants.
PROCEDURE:
Operation Keys:

Key functions:
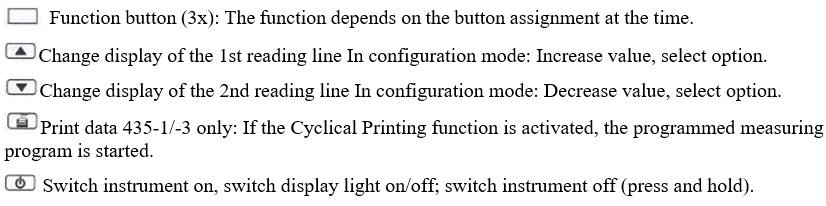
Operating Procedure:
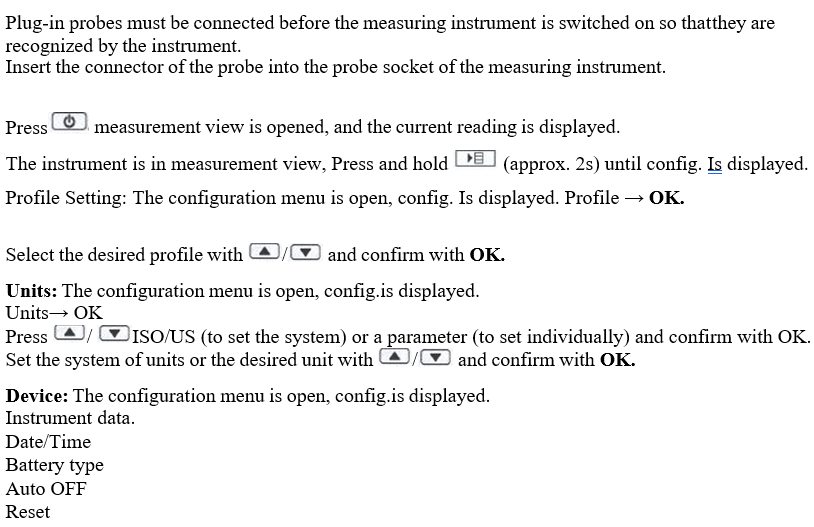
Plug-in probes must be connected before the measuring instrument is switched on so that they are recognized by the instrument.
Pr Min Max
K-factor
Num holes
Click the link to download the Word copy of Procedure: https://pharmaguidehub.com/product/operation-of-anemometer/
Probe : The configuration menu is open, Config.is displayed.
Probe→ OK → Te-Type→ OK.

Language: The configuration menu is open, config.is displayed.
Language → OK
Measuring procedure

Select the Velocity and select “m/s” or “fpm” according to the measuring requirement.
Ensure the white → mark on the sensor head need to face against the direction of air flow.
Hold the probe handle and move slowly towards the airflow source where air velocity to be measured.
The display will show air velocity reading.
Expose the Anemometer Probe below 1 to 2 inch of the air supply.
Hold the probe for few seconds to get the reading stabilized.
Switch to HOLD mode to hold on the reading. Record the reading.
If another measurement has to be taken immediately after the first one, then wait for a few seconds and then expose the Anemometer probe at the required area.

Safety Instructions :
Do not use the measuring instrument and probes to measure on or near live parts.
Always use the measuring instrument properly and for its intended purpose. Do not use force.
Do not expose handles and feed lines to temperatures in excess of 70°C unless they are expressly permitted for higher temperatures.
Temperatures given on probes relate only to the measuring range of the sensors.
Never store the measuring instrument, measuring cells together with solvents and do not use any desiccants.
Click the link to download the Word copy of Procedure: https://pharmaguidehub.com/product/operation-of-anemometer/
REFERENCES:
Not Applicable
ANNEXURES:
Not Applicable
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
- Controlled Copy No. 01 Head Quality Assurance
- Controlled Copy No. 02 Head-Engineering
- Master Copy Quality Assurance Department
ABBREVIATIONS:
USB: Universal Serial Bus.
m/s : Meters per Seconds.
fpm: Feet per minute.
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
Click the link to download the Word copy of Procedure: https://pharmaguidehub.com/product/operation-of-anemometer/

