ABOUT FBP:
MAKE: GLATT SYSTEM PVT. LTD
MODEL NO.: GFB PRO 300
The Fluidized Bed Processor, GFB PRO 300 is used for rapid and efficient granulation/ coating of Pellets. The material to be processed is loaded into the bowl of the GFB PRO 300. The bowl has a fine SS mesh at the bottom. The air used for drying/fluidizing is successively filtered through Pre (EU 4- filter grade, 10 micron rating, 90% efficiency), Fine (EU 7 grade, 3 micron rating, 99% efficiency) and finally through HEPA filter (EU 13 grade, 0.3 micron rating, 99.997% efficiency). Hot, filtered air up to 80oC is passed at high velocity from the bottom of the FBE bowl through the process material. Because of the high velocity of the air the process material is fluidized. The fluidizing air results into a very efficient heat and mass transfers (heating, cooling, drying, granulating, coating).

Key Parameter of Below Page:
Protocol Contents
Find below pages for complete protocol:
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/operational-qualification-protocol-cum-report-for-fluid-bed-processor/
Key Parameter of Below Page:
Introduction – Purpose
Scope
Find below pages for complete protocol:

Key Parameter of Below Page:
Responsibility
Find below pages for complete protocol:
Key Parameter of Below Page:
Training Record
Find below pages for complete protocol:
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/operational-qualification-protocol-cum-report-for-fluid-bed-processor/

Key Parameter of Below Page:
Execution Team
Find below pages for complete protocol:
Key Parameter of Below Page:
Equipment Detail
Find below pages for complete protocol:

Key Parameter of Below Page:
Operational Qualification
Find below pages for complete protocol:

Key Parameter of Below Page:
Operational Qualification
Find below pages for complete protocol:

Key Parameter of Below Page:
Requalification Criteria
Find below pages for complete protocol:
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/operational-qualification-protocol-cum-report-for-fluid-bed-processor/

Key Parameter of Below Page:
Deficiency and corrective action report
Find below pages for complete protocol:

Key Parameter of Below Page:
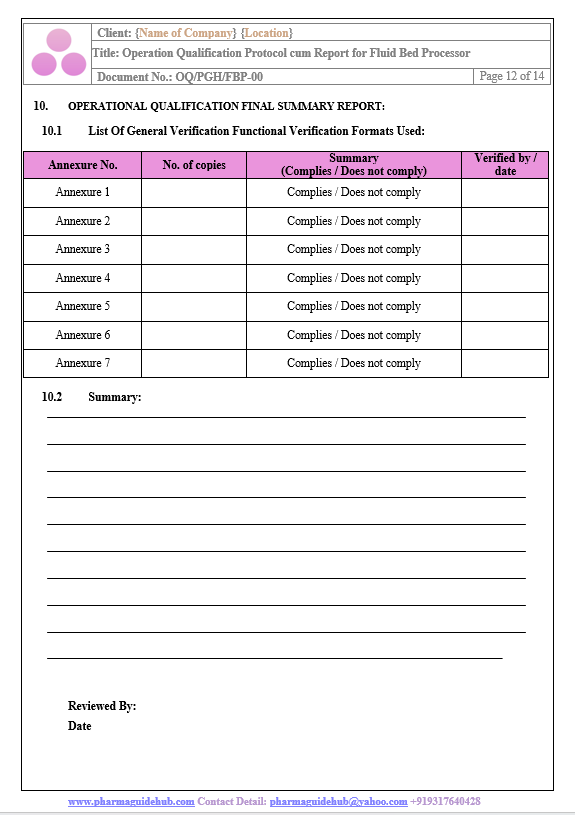
Operational Qualification Final Summary Report:
Find below pages for complete protocol:
For the Execution of Qualification activity at site, Please write us at pharmaguidehub@yahoo.com
Key Parameter of Below Page:
Conclusion
Find below pages for complete protocol:

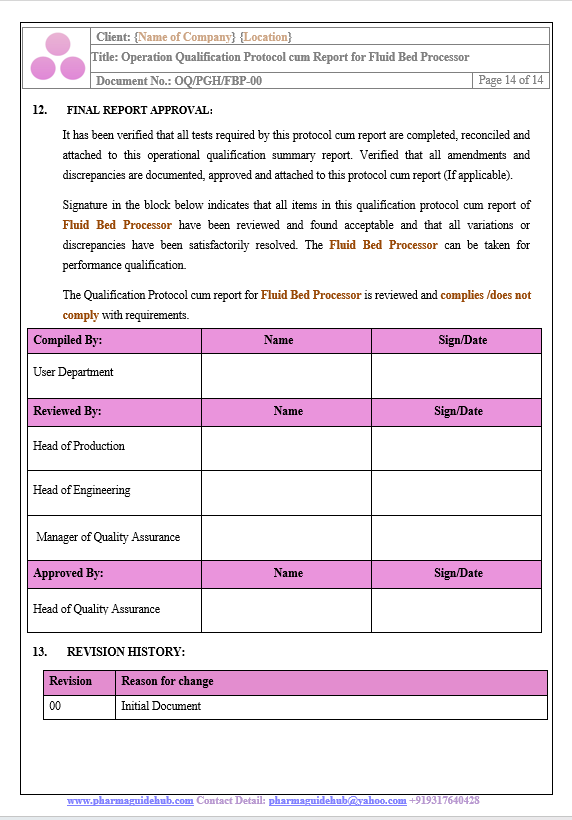
Key Parameter of Below Page:
Final Report Approval
Find below pages for complete protocol:
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/operational-qualification-protocol-cum-report-for-fluid-bed-processor/