About Sejong 77 station Model HRC 77S AWC:
Test: Measuring the noise level emitted by the machine.
The Sejong 77 station Model HRC 77S AWC machine is a high-speed, double-sided tablet press designed for mass production in the pharmaceutical industry. It is capable of producing up to 646,000 single-layer tablets per hour and 184,000 double-layer tablets per hour
The machine features a large 15-inch touch screen for easy operation and monitoring, as well as an Automatic Weight Control (AWC) system for accurate tablet weight control. It also has a disk change system that maximizes productivity and a double-side discharge system for efficient tablet collection.
The HRC 77S is a reliable and efficient tablet press that is ideal for high-volume production of pharmaceutical tablets. It is available in a variety of configurations to meet the specific needs of different customers.
Specification of HRC-S Series
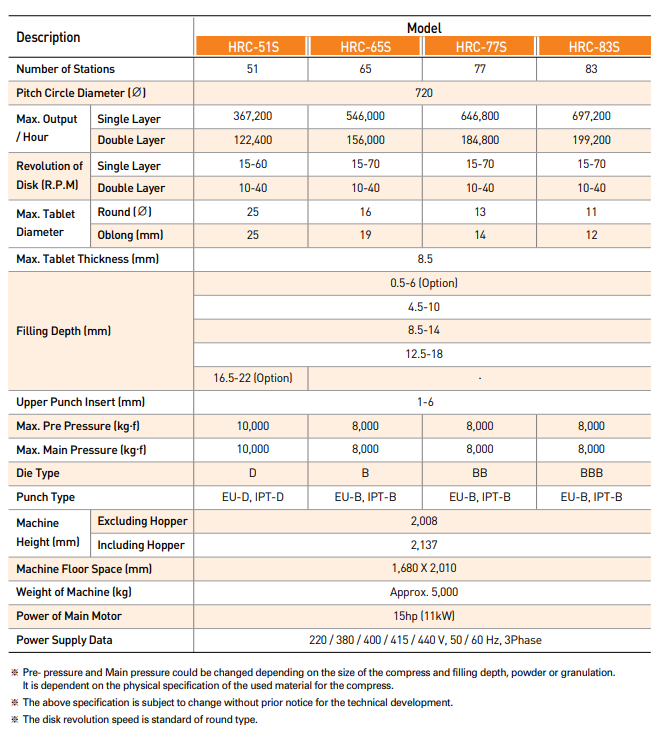
Specification taken from: www.keyinternational.com
Performance Qualification (PQ) is a critical step in ensuring the consistent performance of Sejong 77 Station Model HRC 77S AWC machine. It involves a series of tests to verify that the machine operates within specified parameters and produces tablets that meet predefined quality standards.
Key Parameters for the Sejong 77S AWC Machine for PQ:
Tablet Weight Uniformity:
Test: Weighing a sample of tablets and calculating the weight variation.
Parameter: Percentage of tablets within specified weight limits.
Tablet Hardness:
Test: Measuring the force required to break a tablet.
Parameter: Minimum and maximum hardness limits.
Tablet Thickness:
Test: Measuring the thickness of a sample of tablets.
Parameter: Specified thickness range.
Tablet Friability:
Test: Subjecting tablets to abrasion and measuring weight loss.
Parameter: Maximum allowable weight loss.
Tablet Disintegration Time:
Test: Immersing tablets in a dissolution medium and measuring the time taken for them to disintegrate.
Parameter: Maximum disintegration time.
Machine Efficiency:
Test: Calculating the percentage of time the machine is producing tablets.
Parameter: Minimum efficiency.
Machine Noise Level:
Parameter: Maximum allowable noise level.
Performance Qualification Protocol
Find below pages for completes Performance Qualification protocol:

Key Parameter of Below Page
Its a page of Pharmaguidhub
Find below pages for complete protocol:
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/performance-qualification-protocol-for-sejong-77-station-double-rotary-compression-machine/

Our Knowledge Sharing Platform:
To get regular updates on pharmaceutical industry you can join us at our following knowledge sharing platforms:
- Web address: www.pharmaguidehub.com
- Telegram Link: https://t.me/pharmaguidehub
- LinkedIn: www.linkedin.com/in/pharmaguidehub
- Facebook Page Link: https://www.facebook.com/profile.php?id=61557224322551
- Twitter Link: https://x.com/pharmaguidehub
- WhatsApp Group Link: https://chat.whatsapp.com/HY09XT4iEL07oCE4Am3JUW
Key Parameter of Below Page
Table of Contents:
Find below pages for complete protocol:
Key Parameter of Below Page
Objective
Scope
Find below pages for complete protocol:
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/performance-qualification-protocol-for-sejong-77-station-double-rotary-compression-machine/

Key Parameter of Below Page
Responsibility:
Find below pages for complete protocol:
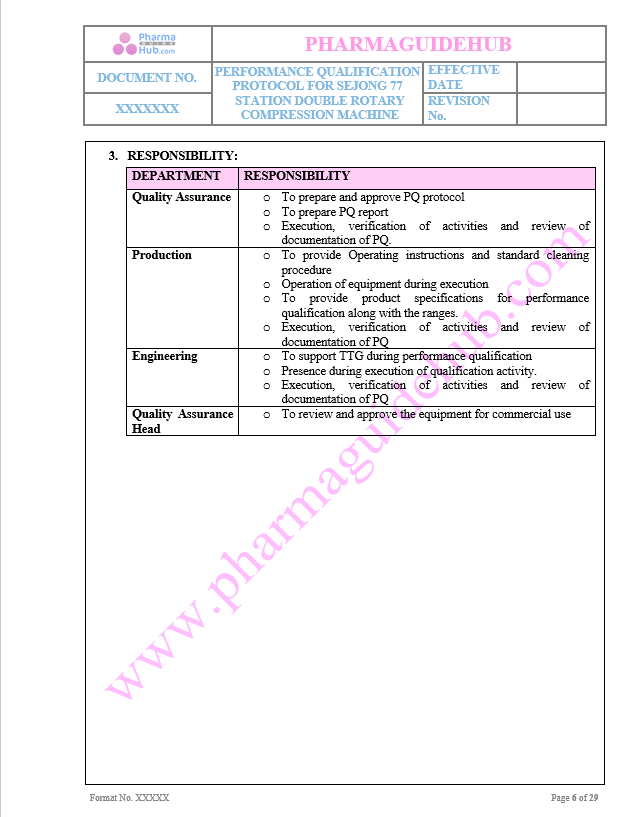
Key Parameter of Below Page
Perforformance Qualification:
Prerequisites
completeFind below pages for completes protocol:

Key Parameter of Below Page
Raw material requirement:
For the execution of PQ required material details are given below
Find below pages for complete protocol:
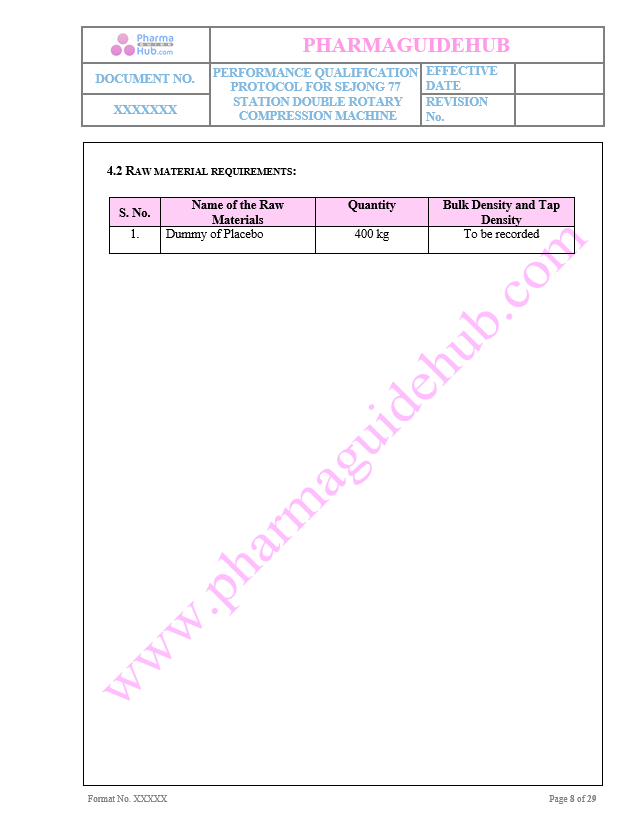
Key Parameter of Below Page
Required Instrument:
For the execution of PQ Required instrument details is given below
Find below pages for complete protocol:

Key Parameter of Below Page
Procedure:
For the execution of PQ required system description is mentioned below
Find below pages for complete protocol:

Key Parameter of Below Page
Major Process stages and configurable parameters detail has given below
Performance verification stratergy given below
Find below pages for complete protocol:
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/performance-qualification-protocol-for-sejong-77-station-double-rotary-compression-machine/
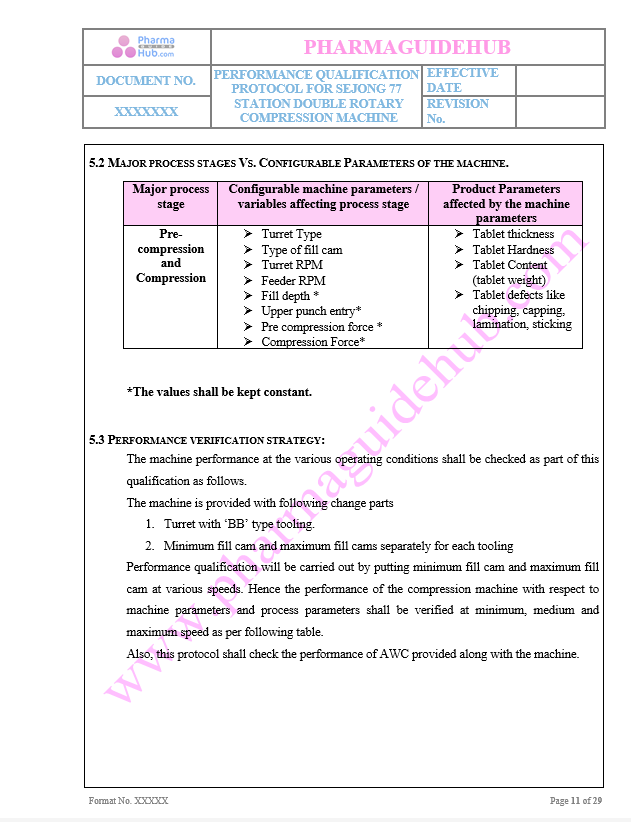
Key Parameter of Below Page
Measurement criteria
Performance verification criteria
Find below pages for complete protocol:
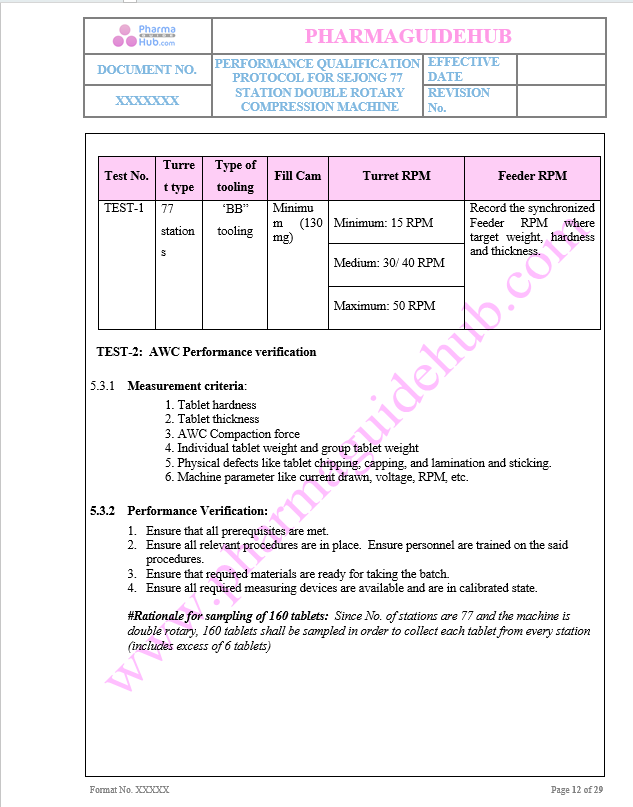
Key Parameter of Below Page
AWC Performance Verification
Acceptance Criteria
Process Parameter
Find below pages for complete protocol:
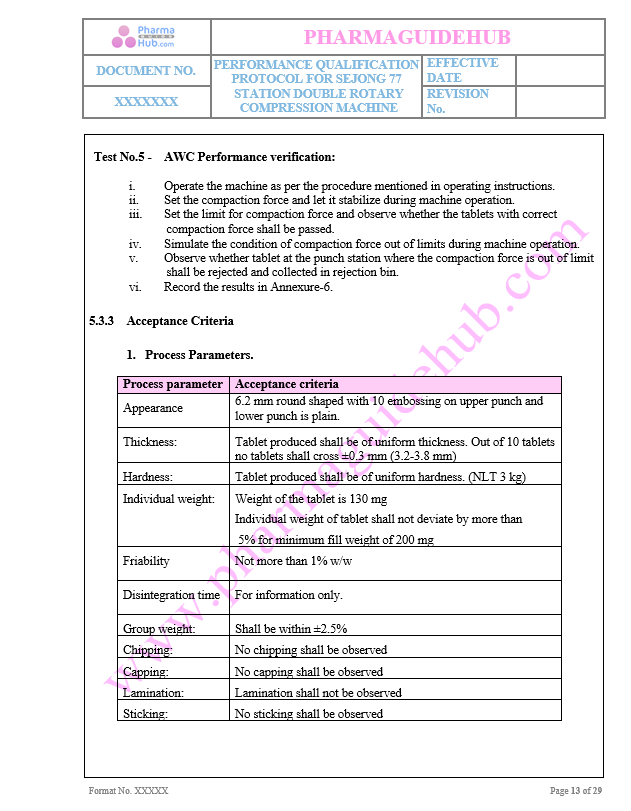
Key Parameter of Below Page
Machine Parameter
Find below pages for complete protocol:

Key Parameters of Motors
Performance of Turret Motor
Find below pages for complete protocol:
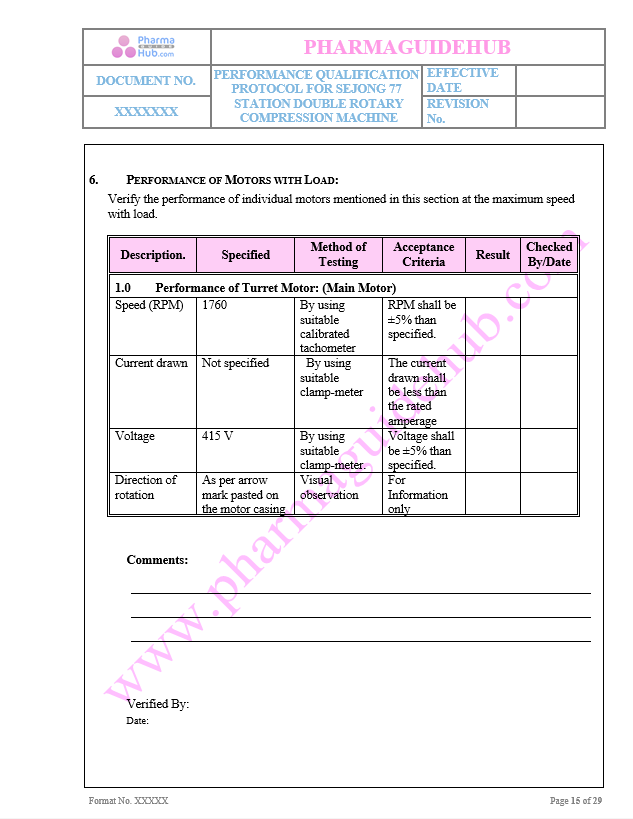
Key Parameters of Motors
Performance of Feeder Motor LHS
Find below pages for complete protocol:
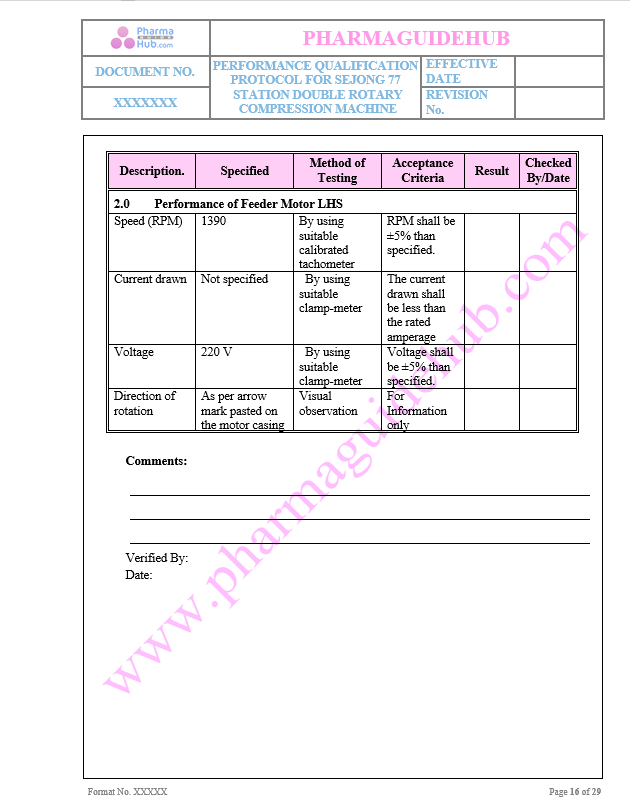
Key Parameters of Motors
Performance of Feeder Motor RHS
Find below pages for complete protocol:
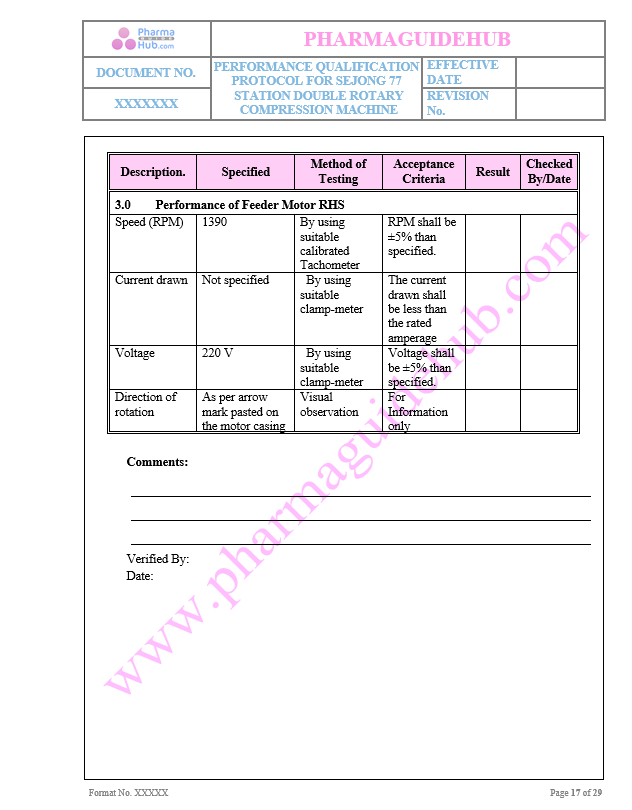
Key Parameters of Motors
Performance of Oil Pump Motor
Find below pages for complete protocol:
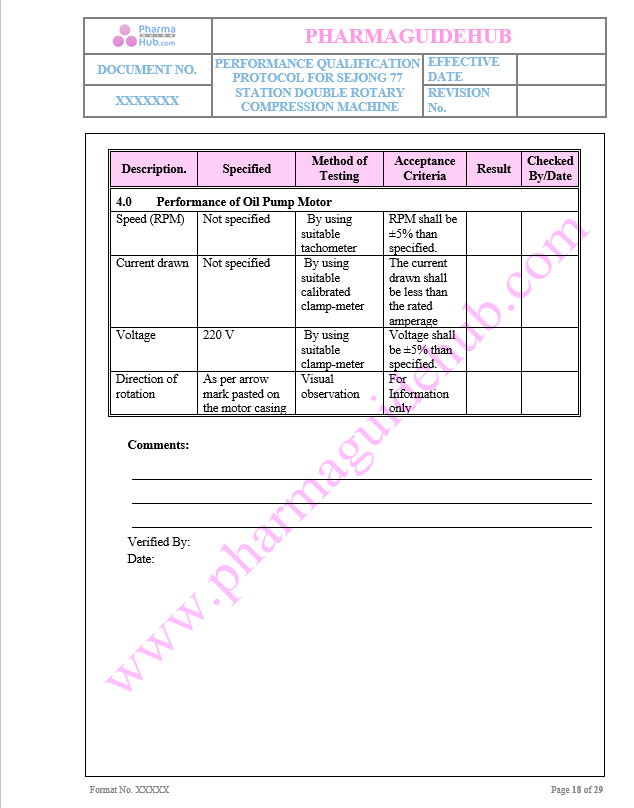
Key Parameter of Below Page
Detail of Instrument Used:
Find below pages for complete protocol:

Key Parameter of Below Page
Deviation from pre-approved Protocol
Actions of deviation from pre approved protocol
Find below pages for complete protocol:

Key Parameter of Below Page
Summary of PQ
Conclusion of PQ
Find below pages for complete protocol:
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/performance-qualification-protocol-for-sejong-77-station-double-rotary-compression-machine/

Key Parameter of Below Page:
Post approval of PQ
Find below pages for complete protocol:
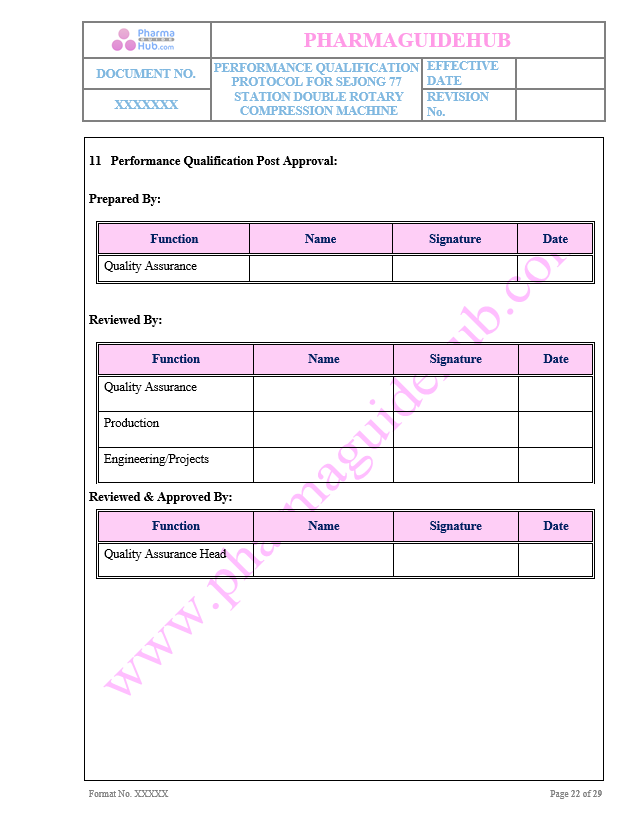
Key Parameter of Below Page:
Tablet Hardness Measurement sheet
Find below pages for complete protocol:
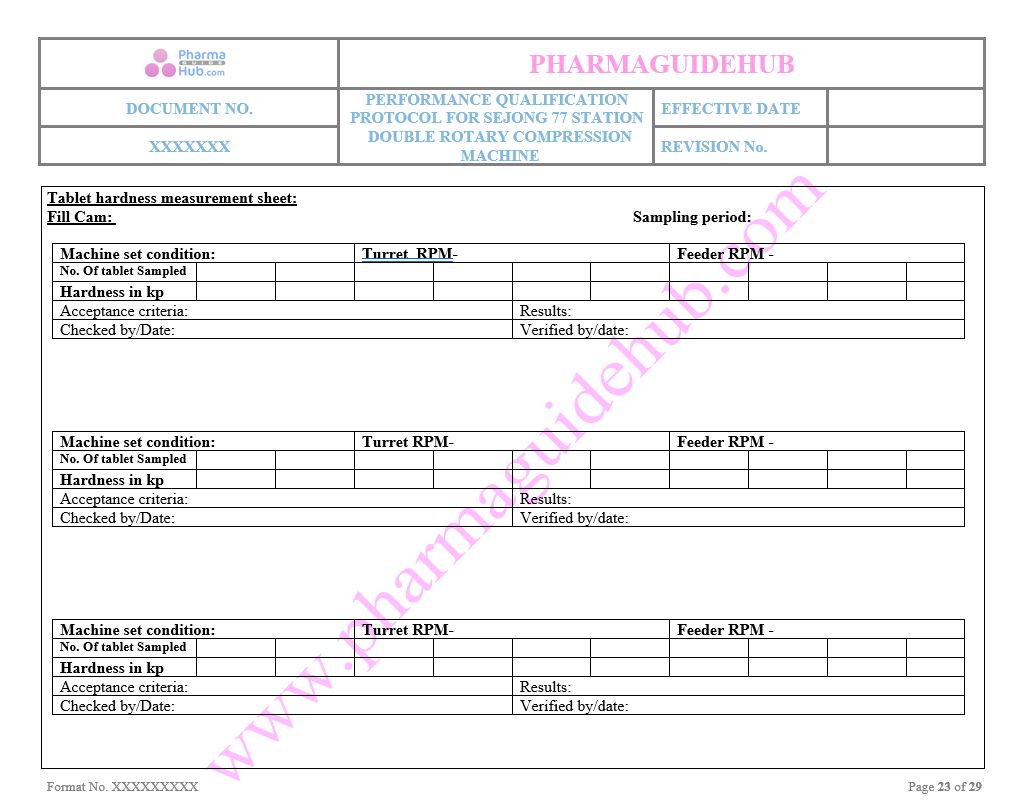
Key Parameter of Below Page:
Tablet Thickness Measurement sheet
Find below pages for complete protocol:
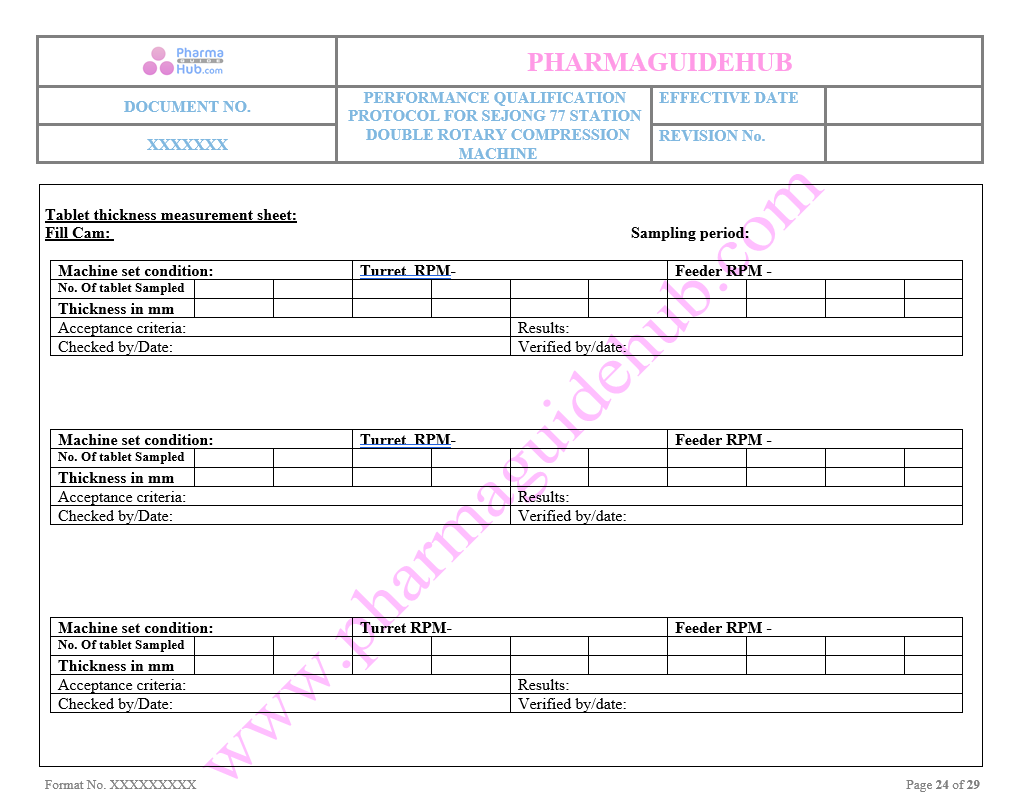
Key Parameter of Below Page:
Individual Tablet Weight Measurement sheet
Find below pages for complete protocol:
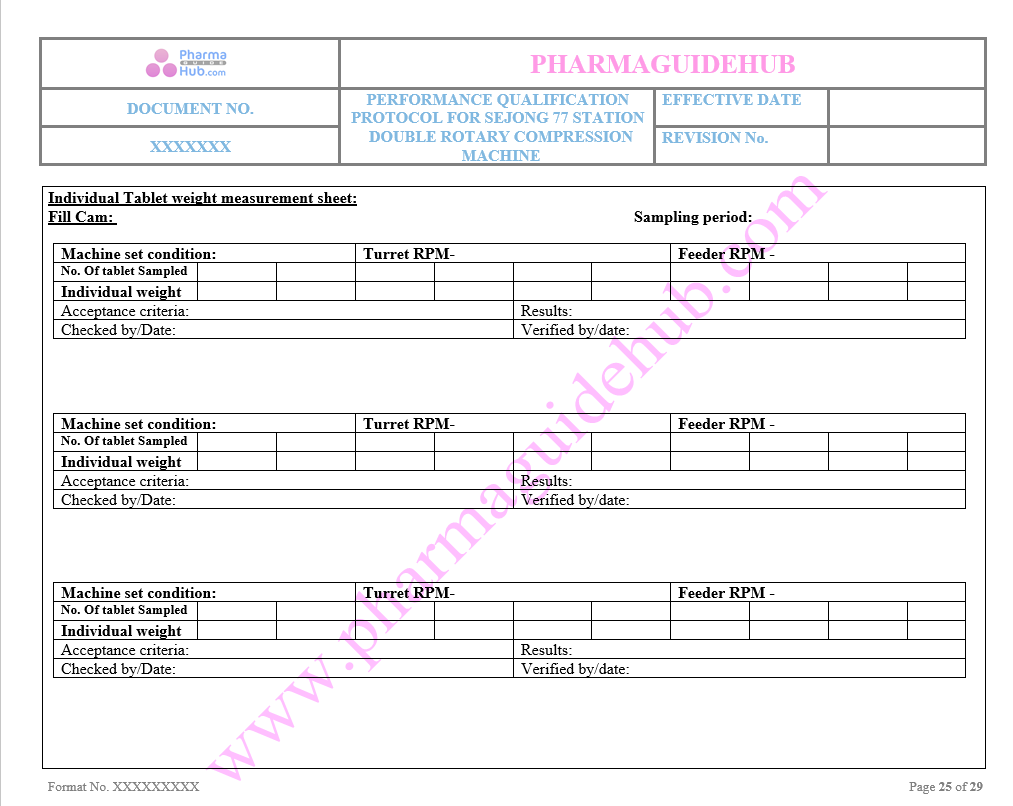
Key Parameter of Below Page:
Individual Tablet Weight Measurement sheet
Find below pages for complete protocol:
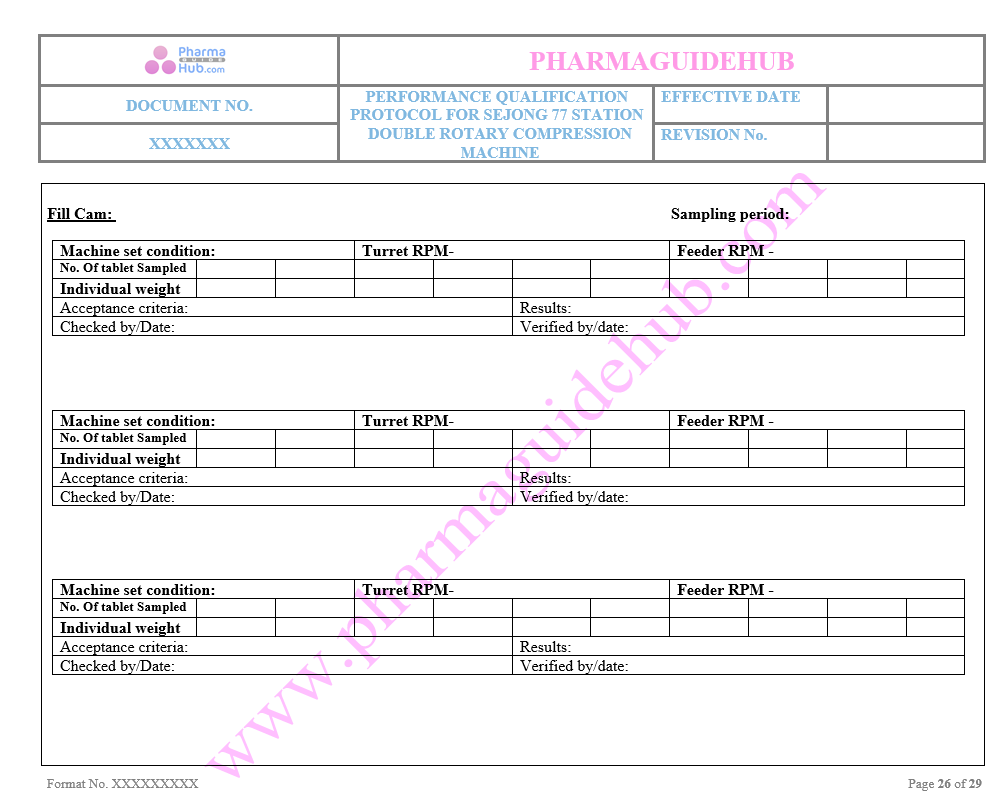
Key Parameter of Below Page:
Group Weight Measurement sheet
Find below pages for complete protocol:
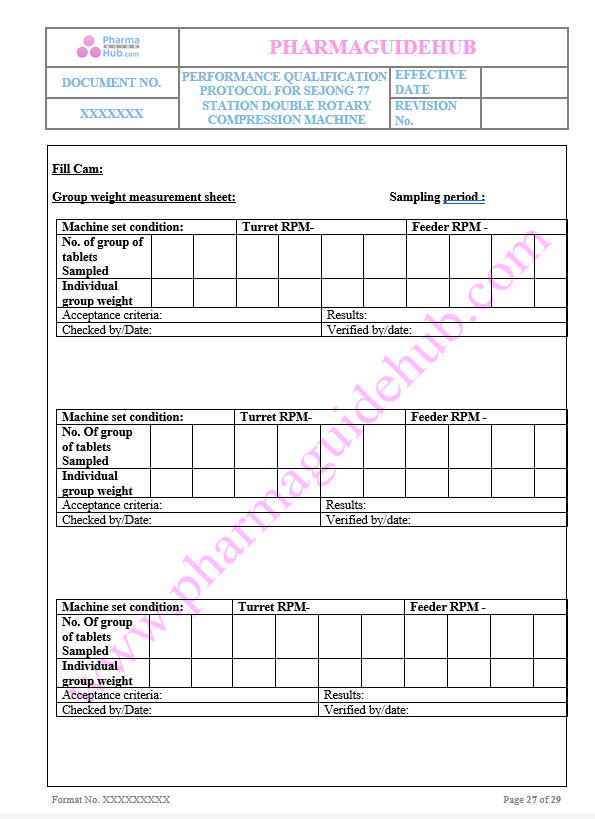
Key Parameter of Below Page:
Tablet Physical Verification sheet
Find below pages for complete protocol:
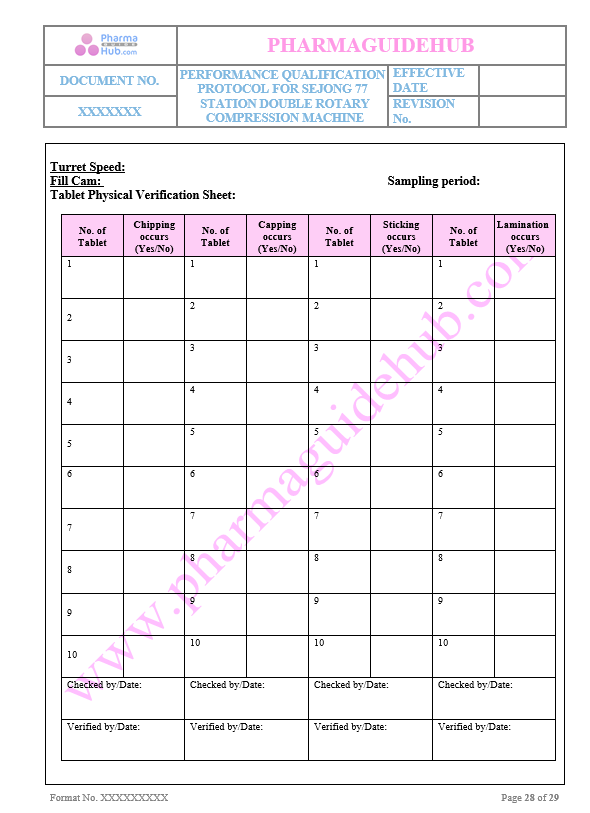
Key Parameter of Below Page:
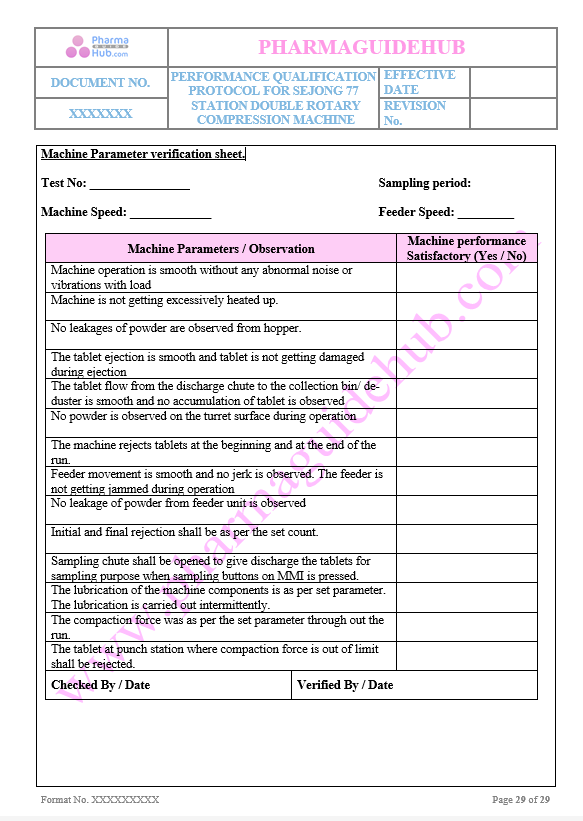
Machine Parameter Verification sheet
Find below pages for complete protocol:
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/performance-qualification-protocol-for-sejong-77-station-double-rotary-compression-machine/



