- PROCEDURE:
- Prepare the volumetric solutions using purified water and A.R. Grade Chemicals, according to the procedures given in respective GTPs.
- Volumetric solutions shall be standardized against primary standards.
- The strengths of volumetric solutions should not differ from the prescribed value by more than 10.0%.
- The standardization should be done in duplicate and the percentage deviation of molarity / normality so obtained should not be more than 0.20%.
- All volumetric solutions should be stored in clean and dry glass bottles.
Note: Light sensitive solutions should be stored in amber glass bottles.
- Solutions of molarity/normality greater than the specified shall be prepared by using proportionate amounts of the reagents. Solutions of lower strengths shall be prepared by doing an exact dilution of the concentrated solution.
Note: Solutions of strengths lower than 0.1M shall be prepared freshly and shall be discarded of after usage.
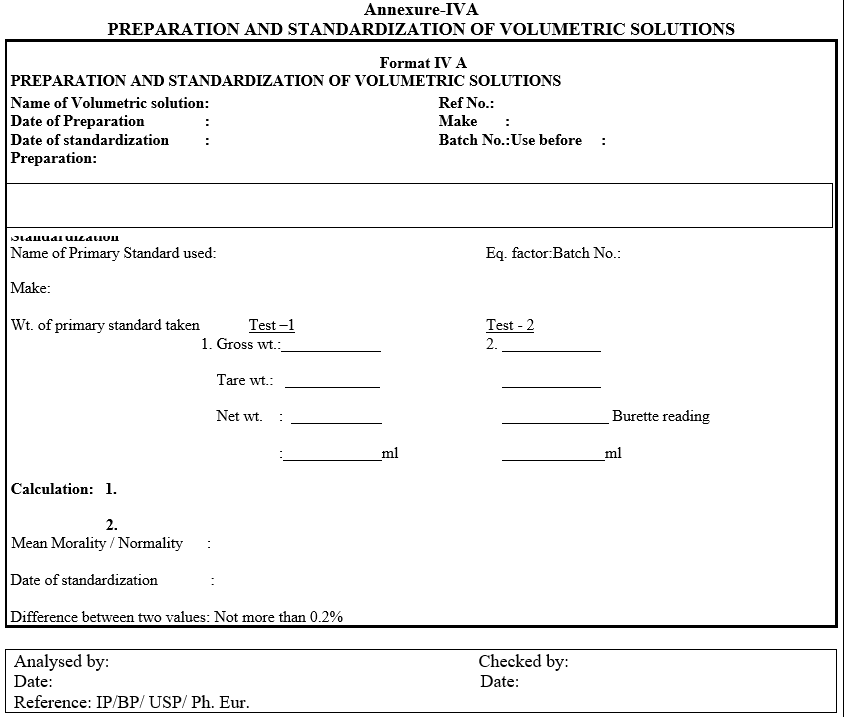
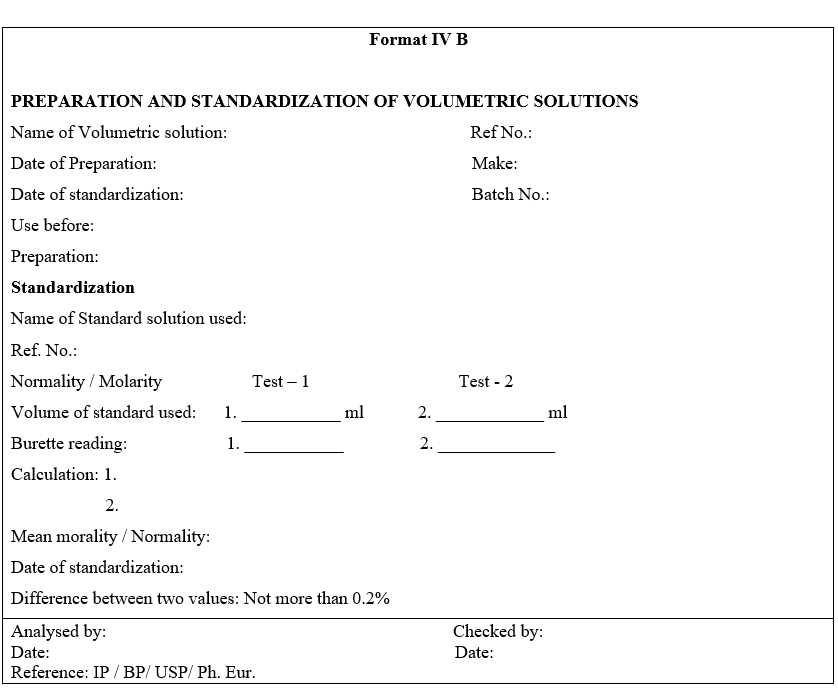
- Label the bottle with information given in Format-I.
- Reference Number is given to each volumetric solution. Example: – For 0.1M Ammonium Thiocyanate the number is VS/01, 01 represents the identity of the volumetric solution.
- AR number is given to each volumetric solution preparation, which is the serial number of that particular volumetric solution. Ex. For 0.1M Ammonium Thiocyanate, the AR number for the initially prepared solution will be VS/01/901, where 01 represents the reference number and 901 is the AR number in which 9 represents the Year and 01 represents the serial number.
- Check the solution before use and discard the solutions showing the evidence of any physical change such as sedimentation, discoloration, crystallization.
- Volumetric solutions should be used within one month from the date of preparation.
- Re-standardize the solutions before use if mentioned in General test procedure. Indicate “Restandardize before use” on the label against strength.Enter all the weights and calculations in the format whichever is applicable.
- Record the details of preparation and standardization in a register.
Note: If commercial grade volumetric solutions are available with COA`s, use the same after Standardization.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Volumetric Solution Label |
| Annexure-II | List of Volumetric solutions |
| Annexure-III | Preparation and standardization Record |
| Annexure-IV | Preparation and standardization of volumetric solutions |
Annexure-I
VOLUMETRIC SOLUTION LABEL

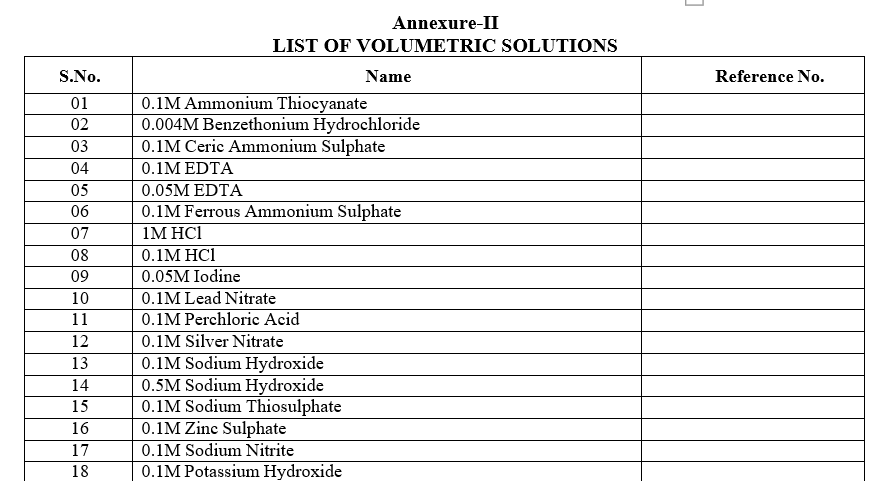
Annexure-II
LIST OF VOLUMETRIC SOLUTIONS
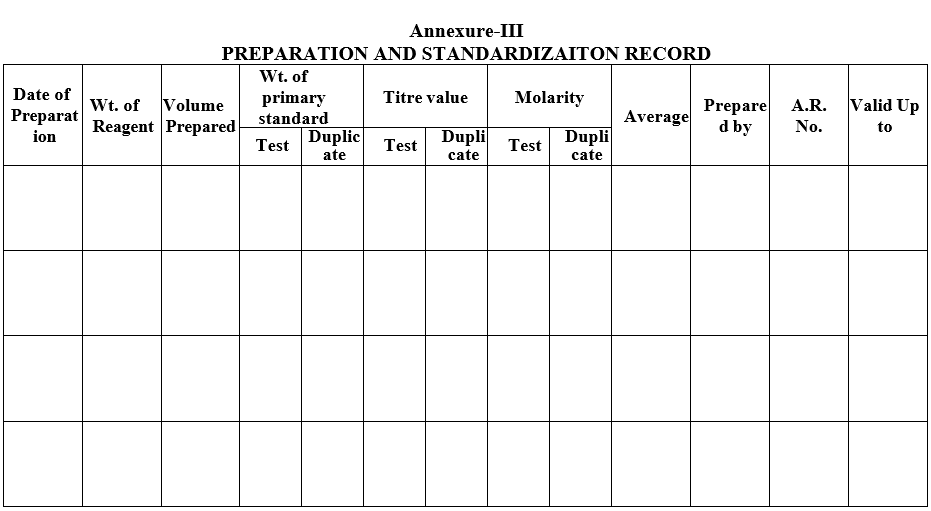
Annexure-III
PREPARATION AND STANDARDIZAITON RECORD
Annexure-IVA
PREPARATION AND STANDARDIZATION OF VOLUMETRIC SOLUTIONS