OBJECTIVE:
To lay down a Procedure for Preparation, Approval, and Revision of Master Formula Record.
- SCOPE:
This SOP is applicable for Preparation, Review, Approval and Revision of Master Formula Record (MFR) for Manufacturing & Packing Process. This SOP is applicable at {Company Name} {Company Location}.
- RESPONSIBILITY:
The responsibility for using this SOP lies to concerned user (Operating Officer / Executive) who initiates the MFR, Operating Manager who checks and reviews the MFR, QA Head who approves and Revise the MFR.
- ACCOUNTABILITY:
QA Head Shall be accountable for implementation of SOP.
- PROCEDURE:
- PREPARATION OF A NEW MFR:
For preparation of MFR, initiators shall follow following Guidelines / Instructions:
- The MFR shall be written in English Language by using Microsoft Word (Times New Roman Font) typing.
- MFR shall be prepared by QA Department on the basis of documents provided by the F&D Department.
- The MFR shall cover all activities of Operation in order and shall be prepared separately for Manufacturing & Packing Process.
- Initiator shall check the completeness of draft MFR and send the hard copy to the Head Production and Head Quality Control for review.
- The reviewer shall review the draft MFR for accuracy of the subject matter & logical sequence of instructions.
- On receipt of the comments (if any), the same shall be reviewed and incorporated in the MFR.
- The draft MFR after completion of checking and incorporation of comments or suggestions as agreed, shall be destroyed, this shall be done in Originating Department.
- Master Copies of all MFR shall be printed in QA Department only.
- MFR should be handled with care and should not be spoiled or torn.
- No manual correction shall be made in Approved Copy.
- Storage of MFR (Master Copy / Soft Copy):
- All Master Copy / Soft Copy of Approved MFR shall be stored in QA Department with Password Protected System and Data Backup shall be kept in Information Technology (IT) Department.
- LAYOUT OF MFR:
- MFR Format Considerations:
- All MFR shall contain Header, Footer and Body. Format for “Master Formula Record” is shown in Annexure-I.
- All pages shall contain Format No. in Footer part.
- All the points in the MFR shall be numbered sequentially and sub paragraph of the MFR be also numbered sequentially with an incremental number derived from the heading number. Bullet Numbering may be use.
- Wherever necessary illustrations and drawing shall be indicated to provide better clarity and understanding of the Process / System.
- MFR shall have Critical Process Parameters with their limits.
- Font size of table Content may be Changeable in case of insufficient space but it should be not less than 8 Font.
- All MFR contents shall be covered by Single Borderline with ½ Line Weight (Width).
- Line Spacing and Font Size: The Line Spacing between two points or title & subtitle shall be 1.5 lines font style shall be Times New Roman 12 font. Font size may be less in case of Table and Remarks.
- MFR (Master Copy) shall be printed on A4 size 75 GSM Paper using “Times New Roman” Font with black Ink. Printing shall be done on one side of the paper only. If required color print can be given.
- Paper Selection for Print: Paper 8.5” (Width), 11.5” (Height), Border Top 24pt,Left 24pt, Right 24pt and Bottom 31pt with A4 Size Scaling.
- CONTENT OF HEADER AND FOOTER:
- Header:
- The Header of MFR shall have the Name of Organization (Including Name of Location).Header shall have the “Logo” of Organization in Left corner on Top and Master Formula Record with Product Name in centerwritten in Bold and Capital letter of font size 12 as per format shown in Annexure-I
- MFR No.: Shall be written in Bold and Capital Letter of Font Size 12.
- MFR Numbering System:
- Master Formula RecordNumber for Manufacturing Process / Packing Process shall be allocated as MFR/XXXX-RR.
- Where:
| “MFR” | : | Master Formula Record |
| “/” | : | Separator |
| “XXXX” | : | 4 DigitProduct Code |
| “-” | : | Hyphen |
| “RR” | : | Revision Number |
Example: MFR/4000-00
- MFR Numbering System (Loan License Product):
Master Formula RecordNumber for Manufacturing Process / Packing Process (for Loan licence Product shall be allocated as MFR/XXXXX-RR.
Where:
| “MFR” | : | Master Formula Record |
| “/” | : | Separator |
| “XXXXX” | : | 5 DigitProduct Code |
| “-” | : | Hyphen |
| “RR” | : | Revision Number |
Example: MFR/40000-00
- Effective Date: Effective Date shall be assigned in the form of DD/MM/YY.Shall be written in bold and Normal Letter of Font Size 12.
- Page No.: Shall be written in Bold and Normal of Font Size 12. The Page Number shall be mentioned in ‘X of Y’ format. For Example: If a MFR contains 60 pages then the first Page of the MFR shall be 1 of 60 and the second page shall be 2 of 60 respectively.
- Footer:
- Format No.: 09 Normal & Capital font size printed on the left corner of the page after Footer, out of page border and shall be printed as per Annexure-I (For Manufacturing Process) & Annexure-III (For Packing Process) on all pages of the MFR.
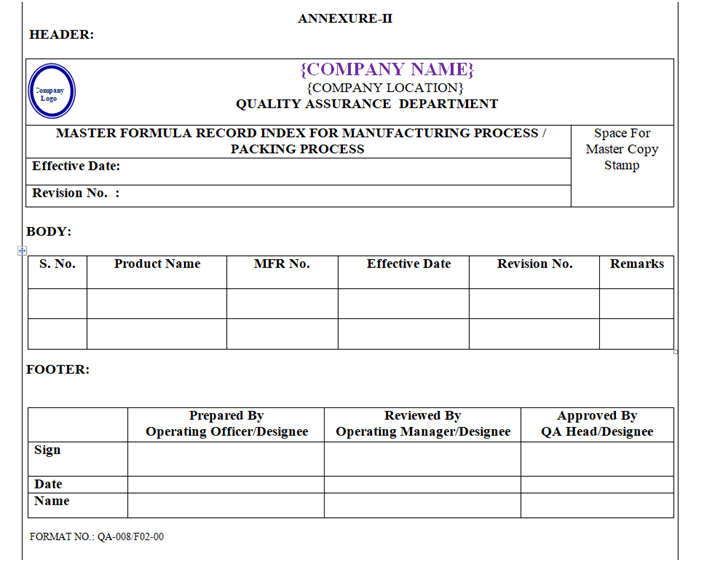
- A Master List of MFR shall be maintained by QA Department as per Annexure-II.
- The list of MFR shall be updated by QA once in a year or whenever required, manual corrections shall not be allowed in this list, and the list shall be converted into protected PDF format with print option only.
In case of discontinuation of the existing MFR same MFR No. shall not be allotted to any newly prepared MFR.
- CONTENT OF THE MASTER FORMULA RECORD:
- First Page: First page shall contain Master Formula Record with Product Name as per format shown in Annexure-I.
- MFR Supersede No.: Shall be written in Bold and Normal Letter of Font Size 12. Supersedes shall have the History of the previous Revision No. and shall start from Nil.
- Market Domestic/ India. Shall be written in Bold and Normal Letter of Font Size 12.
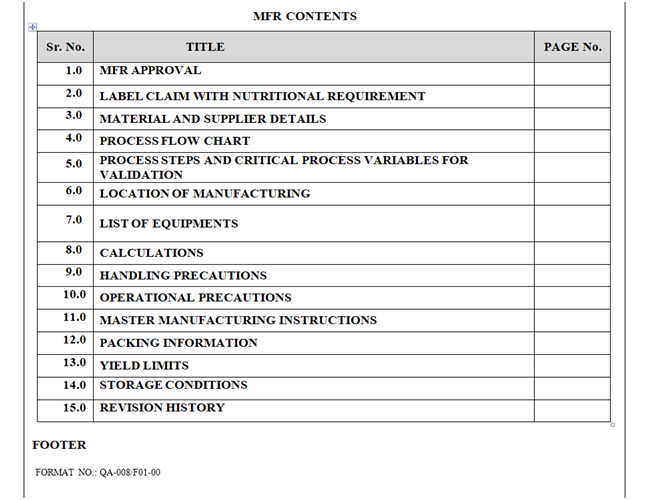
- Second Page of MFR: Second Page of MFR shall contain “TABLE OF INDEX” in the form of given table.
TABLE OF INDEX
| S. No. | Title | Page No. |
- Third Page of MFR: Third Page of MFR shall be Approval Page and contain following Headings in the given form.
PREPARED BY:
| COMPANY NAME | NAME | DESIGNATION & DEPARTMENT | SIGN/DATE |
| {COMPANY NAME} |
REVIEWED BY:
| COMPANY NAME | NAME | DESIGNATION & DEPARTMENT | SIGN/DATE |
| {COMPANY NAME} |
APPROVED BY:
| COMPANY NAME | NAME | DESIGNATION & DEPARTMENT | SIGN/DATE |
| {COMPANY NAME} |
- Label claim with nutritional requirement: Label claim with nutritional requirement shall be prepared as per given table.
| S. No | Material Code | Name of Ingredient | Vendor details | Grade of ingredient | Specification No. | Category | Quantity per 100 g | Quantity per 100kg | ||
| UOM | Quantity | UOM | Quantity | |||||||
- Material and supplier details: Material detail shall be prepared as per given table.
| S. No. | MATERIAL NAME | SUPPLIER NAME |
- Process Flow chart: Manufacturing Process shall be added in process chart.
- Process steps and critical process variables for validation: All process stapes and critical process parameter shall be added.
- Location of manufacturing
- List of equipments: List of equipment shall be prepared as per given table
| S. No. | Equipment Name | ID No. |
- Calculation
- Handling precautions
- Operational precautions
- Master manufacturing instructions: (include every individual stage)
- Yield limits
- Storage conditions
- Revision history:
| Revision No. | Revision for Revision | Details of Revision | Effective Date |
- FONT STYLE / SIZE OF HEADER, FOOTER & BODY CONTENTS :
| NAME OF CONTENT | FONT SIZE |
| HEADER | |
| Name of the Organization | 12 Bold & Capital |
| Logo with {Company Name} (On Left Hand Side Corner of The Page) | Logo– Height-0.75’’& Width-0.65’’ |
| Master Formula Record with Product Name | 12 Capital & Bold |
| MFR No. | 12 Normal & Bold |
| Revision No. | 12 Normal & Bold |
| Effective Date | 12 Normal & Bold |
| Page | 12 Normal & Bold |
| FOOTER | |
| Format No. | 09 Capital & Normal |
| BODY (First Page): | |
| Master Formula Record | 28 Bold & Capital |
| Product Name | 28 Normal & Bold |
| MFR Supersedes No. | 12 Normal & Bold |
| Market | 12 Normal & Bold |
| Shelf Life | 12 Normal & Bold |
| BODY (Second Page): | |
| Table of Content (Heading) | 14 Capital & Bold |
| Table Header and other Table Content | 12 Normal & Bold |
| Table Sub Headings | 12 Capital Normal & Bold |
| BODY (Third Page): | |
| All Information | 12 Capital & Bold |
| Other Pages information of Body Part: | |
| Headings | 12 Capital & Bold |
| Sub Headings | 12 Normal & Bold |
| Other Information | 12 Normal |
| Table Heading and Header of the Table | 12 Capital / Normal & Bold |
| Table Body Part Content | 12 Normal |
- GENERATION OF MASTER COPY:
- MFR (Master Copy) shall be printed on A4 size 75 GSM Paper using “Times New Roman” Font with black Ink. Printing shall be done on one side of the paper only. If required color print can be given.
- Master Copy of MFR shall be stamped as ‘MASTER COPY’ in Red ink below Right Upper Corner of the page on all the pages.
- Any change in MFR shall be done after Approval of Change Controlin both Soft and Hard Copy.
- REVISION OF MFR:
- Any change in MFR shall be done only after Approval of Change Control.
- The “Change Control” form shall be issued by QA after recommendation of QA Head. After receiving filled Change Control from initiating department, QA Head shall approve the proposal on the basis of satisfactory Scientific Rationale.
- After Approval of Change Control QA Officer / Executive shall incorporate the changes in soft copy. QA Head shall approve the revised MFR in hard copy and shall be approved.
- Previous Master Copy shall be stamped as “OBSOLETE COPY” and Soft Copy of obsolete MFR shall be stored in a separate folder.
- The approved revised hard copy of MFR shall be treated as “MASTER COPY”.
- Obsolete MFR shall be retained till the life of the organization.
- REFERENCES:
- A WHO Guide to Good Manufacturing Practice (GMP) Requirements, Part–I, Standard Operating Procedures and Master Formulae, WHO/VSQ/97.01
- WHO Technical Report Series 961.
- ANNEXURES:
| ANNEXURE No. | ANNEXURE TITLE | FORMAT No. |
| Annexure-I | Master formula Record | QA-008/F01-00 |
| Annexure-II | Master Formula Record Index for Manufacturing Process / Packing Process | QA-008/F02-00 |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Quality Assurance Head
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| SOP | : | Standard Operating Procedure |
| No. | : | Number |
| Ltd. | : | Limited |
| QA | : | Quality Assurance |
| MFR | : | Master Formula Record |
| S. No. | : | Serial Number |
| IT | : | Information Technology |
| MFR No. | : | Master Formula Record Number |
| Mfg. Date | : | Manufacturing Date |
| Exp. Date | : | Expiry Date |
| MRP | : | Maximum Retail Price |
| ID No. | : | Identification Number |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be added manual |
ANNEXURE- I

ANNEXURE – II