OBJECTIVE:
To lay down the procedure for preparation, receipt, issue, labelling and usage of hand disinfectants.
SCOPE:
This SOP is applicable to the procedure for preparation, receipt, issue, labelling and usage of hand disinfectants at {Company Name} {Location}.
RESPONSIBILITY:
- All employees working on Premises is responsible to follow the procedure as per SOP.
- Head HR – is responsible for compliance of the SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
Key Points:
Hand disinfectants, a staple in the pharmaceutical industry, are crucial for maintaining hygiene and preventing the spread of infections. These products, typically formulated as gels, liquids, or wipes, contain active ingredients like alcohol or antimicrobial agents that effectively kill bacteria and viruses on the skin’s surface. They are widely used in healthcare settings, food processing facilities, and public spaces to reduce the risk of contamination and promote a clean environment. Proper hand hygiene practices, including the regular use of hand disinfectants, play a vital role in safeguarding public health.
PROCEDURE:
Receipt and Issue:
On the receipt of disinfectants and cleaning agents all the relevant documents shall be verified by in charge engineering stores (approved vender and excise particulars, purchase order).
All disinfectants and cleaning agents shall be received by the engineering warehouse, containers /bottles shall be cleaned with using dry lint free cloth.
The warehouse personnel will inform to QC department as per the SOP.
QC personnel shall review the vender COA against the container /consignment details for description of the product, manufacturing date, expiry date and Batch number etc. and release the same based on vender COA.
Approved labels shall be issued by Quality control department as per the details mentioned in the MRR by following as per the “Receipt, Issuance of Chemicals and Lubricants”.
Engineering stores will issue the required quantity of disinfectants & cleaning agents as per the request made by the administration & housekeeping department.
Record shall be maintained for consumption of the disinfectants and cleaning agents.
Disinfectants and cleaning agents – Sterillium, Bacillocid Special, Korsolex rapid Isopropyl alcohol, Sokrena, Lysol, Super lime away, Dettol, Allklean, Laundrex EML, Laundrex – Rendent Wash. Etc;
PREPARATION AND LABELLING:
Preparation and usage of 70% IPA solution:
Use purified water for the preparation of 70% IPA.
Housekeeping personnel shall prepare the hand disinfectant (70% IPA solution) in housekeeping wash area.
Frequency: once in a week or whenever required, after preparation of 70% IPA shall be use within one week, after one week remaining quantity shall be disposed and again freshly prepare new solution for next week usage.
The required quantity of IPA shall be taken from production department through miscellaneous request and dilute the same with purified water.
The required quantity of IPA & purified water shall be measure by using the measuring cylinder as per below example.
Example: 7.0 liter of IPA & 3.0 liter of water shall be taken for preparation of the 70%IPA.
Pour the calculated quantity of IPA and purified water into the clean HDPE container. stir the solution properly by using clean aluminum pipe for 5 minutes.
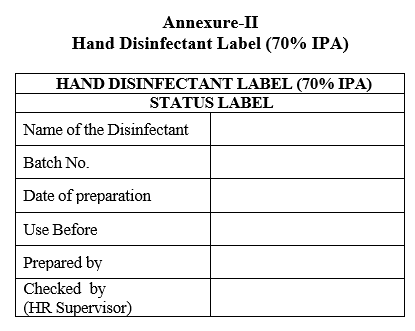
Closed the lid of the HDPE container, check that 70% IPA status label is affixed on HDPE container as well as each spray unit and enter the details in Format.
Refill the hand disinfectant dispenser in all areas check with 70% IPA on first day of every week and whenever required.
HR person shall check the content of the each disinfectant spray unit at least once in a shift.
Top up the same to its capacity with the already diluted IPA (or) ready to use solution Sterillium as per the frequency.
Clean external surface spray unit with lint free cloth & check appropriate of states label, Ensure that spray unit is functioning properly without leakage.
The frequency of rotation the hand disinfectant is once in a month (30 days + (or) – two days).
Rotate the hand disinfectants in such a way that two hand disinfectants are covered in every two months.
For example: Use Sterillium – Fist month Use 70% IPA – Second month Use Sterillium – Third month Use 70% IPA – Fourth month. Repeat the above cycle frequently for all months.
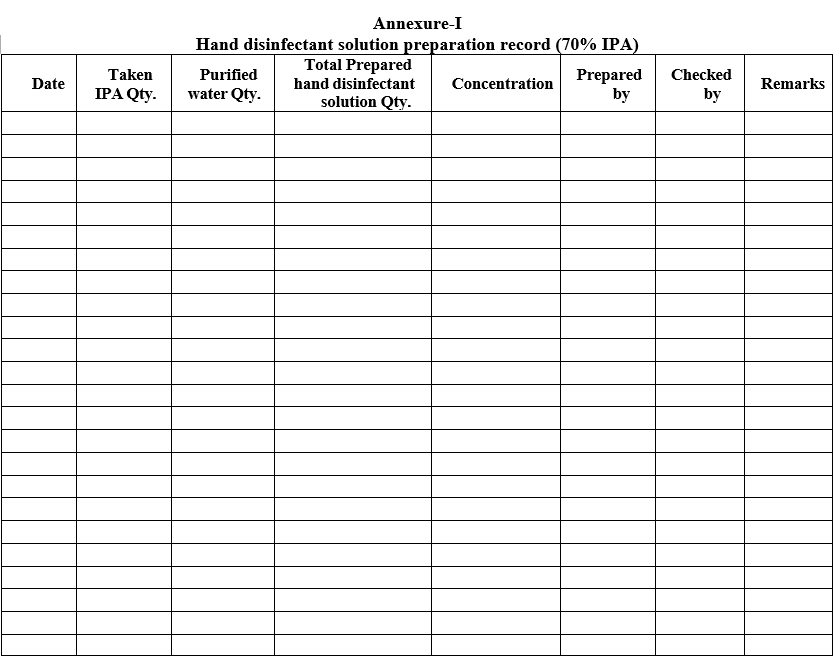
Enter the IPA dilution details as per Format-I.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/preparation-labelling-and-usage-of-hand-disinfectants/
Hand disinfectant Sterillium solution:
Sterillium hand disinfectant is available for ready to use solution, as per the mentioned details of mother container like; manufacturing date, expire date, Batch No should be followed the every spray unit’s in all process areas.
HR person shall check the content of the each disinfectant spray unit at least once in a shift. Top up the same to its capacity with Sterillium solution.
Clean external surface Sterillium spray unit with lint free cloth & check appropriate of states label, ensure that spray unit is functioning properly without leakage.
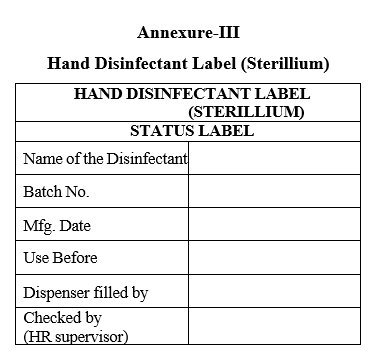
Check that Sterillium solution status label is affixed on each spray unit as per the below label.
Hand disinfectant procedure during entry:
Spray the disinfectant on the hands by pressing the lever of the disinfectant container.
Wet the hands on both sides. (front and backside)
Rub the hands till hands are dry.
Enter into the process area.
REFERENCES:
Not Applicable
ANNEXURES:
| Annexure No. | Title of annexure |
| Annexure-I | Hand disinfectant solution preparation record (70% IPA) |
| Annexure-II | Hand Disinfectant Label (70% IPA) |
| Annexure-III | Hand Disinfectant Label (Sterillium) |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Human resources
- Master Copy : Quality Assurance Department
ABBREVIATIONS:
| HR | : | Human resources |
| IPA | : | Isopropyl alcohol |
| HDPE | : | High density polyethylene |
| COA | : | Certificate of analysis |
| MRR | : | Material receipt request. |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
Hand disinfectant solution preparation record (70% IPA)
Annexure-II
Hand Disinfectant Label (70% IPA)
Annexure-III
Hand Disinfectant Label (Sterillium)
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/preparation-labelling-and-usage-of-hand-disinfectants/


