- OBJECTIVE:
To lay down a Procedure for Preparation of Authorized Personnel List in Working Area.
- SCOPE:
This SOP is applicable for preparation of authorized personnel list in working area of all departments at {Company Name} {Company Location}.
- RESPONSIBILITY:
- All department personnel – Follow the instruction as per procedure.
- QA Sr. Executive-Review and technical correction of SOP.
- ACCOUNTABILITY:
QA Head shall be accountable for implementation of SOP.
- PROCEDURE:
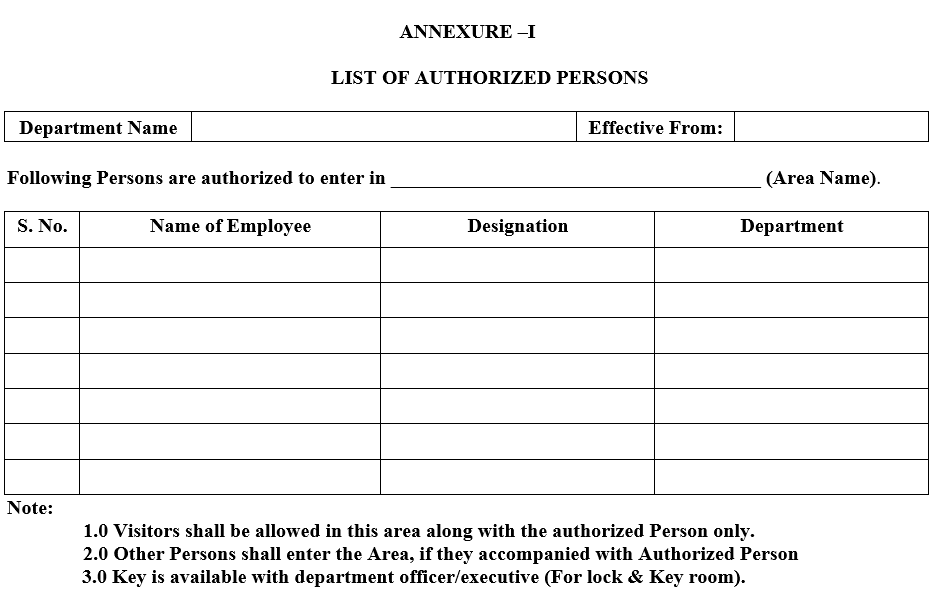
- Concerned Department officer/executive shall prepare a list of Authorized Persons as recommended by Concerned Department Head as per Annexure-I.
- Concerned Department Head/Designee shall check the authorized person list as per requirement.
- QA head/Designee shall review and approved the authorized person list.
- Visitors shall allow in the area along with the authorized person only.
- Microbiologists are authorized to enter in all Critical Areas for Environmental Monitoring.
- Unauthorized person shall not be entering in the area.
- QA officer/Designee or Concerned Department officer/Designee shall update the Authorized Persons List on yearly basis or whenever required.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/preparation-of-authorized-person-list-in-working-area/
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
| Annexure- I | List of Authorized Persons |
- DISTRIBUTION:
| Controlled Copy No. 01 | : | Head Quality Assurance |
| Controlled Copy No. 02 | : | Head Quality Control |
| Controlled Copy No. 03 | : | Head Production |
| Controlled Copy No. 04 | : | Head warehouse |
| Controlled Copy No. 05 | : | Head Engineering |
| Controlled Copy No. 06 | : | Head Human Resources |
| Master Copy | : | Quality Assurance Department |
- ABBREVIATIONS:
| SOP | : | Standard Operating Procedure |
| No. | : | Number |
| Ltd. | : | Limited |
| Lab. | : | Laboratory |
| QA | : | Quality Assurance |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
ANNEXURE –I
LIST OF AUTHORIZED PERSONS
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/preparation-of-authorized-person-list-in-working-area/
Frequently Asked Questions?
1. What is an Authorized Person List (APL)?
Answer: An APL is a formal document maintained by a pharmaceutical company that names all individuals allowed to perform specific critical tasks within controlled working areas. These tasks typically involve manufacturing, testing, releasing, or otherwise handling drug products and materials. Being on the APL signifies trust and competence in adhering to Good Manufacturing Practices (GMP) and other regulatory requirements.
2. Who needs to be on the APL?
Answer: The specific personnel included on the APL can vary depending on the company’s operations and regulatory requirements. Generally, it includes personnel involved in:
- Manufacturing: Production operators, batch record reviewers, equipment operators, cleaning personnel.
- Quality Control: Analysts, microbiologists, QC supervisors.
- Quality Assurance: Auditors, validation specialists, deviation reviewers.
- Packaging: Line operators, label review personnel.
- Warehousing and Distribution: Personnel handling finished product release and shipment.
3. What criteria are used to qualify for the APL?
Answer: Eligibility for the APL depends on several factors, including:
- Education and training: Relevant technical qualifications and specific training in GMP principles and procedures.
- Experience: Demonstrated competence in performing assigned tasks with accuracy and adherence to regulations.
- Security clearance: Background checks and verification of identity and qualifications.
- Health status: Meeting fitness-to-work requirements, including absence of communicable diseases.
4. How are personnel added or removed from the APL?
Answer: A formal process should be established for adding and removing personnel from the APL. This typically involves:
- Application and review: Submission of qualifications and relevant documents followed by assessment by a designated committee.
- Training and orientation: Completion of company-specific training on SOPs, safety procedures, and relevant aspects of GMP.
- Approval and documentation: Official inclusion in the APL with documented justification and records maintained.
- Removal procedures: Established mechanisms for removal based on non-compliance, resignation, or other relevant factors.
5. What are the responsibilities of personnel on the APL?
Answer: Individuals on the APL have a crucial role in maintaining product quality and regulatory compliance. Their responsibilities may include:
- Following established procedures and SOPs (Standard Operating Procedures) meticulously.
- Reporting any deviations or potential quality issues promptly.
- Maintaining accurate and complete records of their work.
- Participating in ongoing training and refresher courses.
- Adhering to strict hygiene and safety practices.
6. What are the consequences of unauthorized access to controlled areas?
Answer: Unauthorized access to controlled areas poses a serious risk to product quality and regulatory compliance. It can lead to contamination, errors, and potential product recalls. Companies typically have disciplinary procedures in place for personnel who violate access control guidelines.
7. How is the APL maintained and updated?
Answer: The APL should be a dynamic document kept current with any changes in personnel, qualifications, or company operations. Regular reviews and updates are essential to ensure its accuracy and effectiveness.
8. What are the differences between APLs in different countries?
Answer: Regulatory requirements and specific APL practices can vary depending on the country and region. Companies operating in multiple markets should be familiar with the relevant regulations and ensure their APL complies with all applicable standards.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/preparation-of-authorized-person-list-in-working-area/





