- OBJECTIVE:
To lay down a Procedure for Microbiological Analysis of Packing Material.
- SCOPE:
This SOP is applicable to the Procedure for Microbiological Analysis of Packing Material in Microbiology Department at {Company Name} {Location}.
- RESPONSIBILITY:
Microbiologist / Executive-Microbiology
- ACCOUNTABILITY:
Manager Quality Control
- PROCEDURE:
- Sampling of Packing Material:
- Before starting the sampling, switch on the blower for 15 minutes. After 15 minutes, wipe the LAF work bench with70% IPA and then start the Primary Packaging Material sampling. After completion of work, clean the work station with70% IPA.
- Bring the sample to microbiology department for testing.
- Carry out the test under Laminar Air Flow.
- Analysis:
- For bottles:
- Fill approximately half of the total volume of bottles with sterile Buffered Sodium Chloride Peptone solution. Shake bottles in such a way that internal surface of bottle are to be rinsed properly.
- Pipette out 1 ml of rinsed solution into four labeled Petri plates. Add approximately 15-20 ml of Soyabean Casein Digest Agar into two plates and Sabourauds Dextrose Agar into two other plates.
- Gently swirl plates to mix sample and medium. Allow to solidify.
- Incubate the plates of Soyabean Casein Digest Agar at 30-35°C for 3 days and Sabourauds Dextrose Agar at 20-25°C for 5 days.
CPP Plain 75 MM & CPP Plain 80 MM, Base Foil and Printed Foils :
- Prepare sterilized swab in Buffered Sodium Chloride PeptoneSolution and take swab of 5×5 cm area from the internal surface of Aluminum foil, PVC film, PVDC film with the help of sterilized 5×5 cm template in such a way total 5×5 cm area to be covered properly.
- Deep swab stick in buffered Sodium Chloride Solution tube and vortex it properly.
- Pipette out 1 ml of rinsed solution into four labeled Petri plates. Add approximately 15-20 ml of Soyabean Casein Digest Agar into two plates and Sabourauds Dextrose Agar into two other plates.
- Gently swirl plates to mix sample and medium. Allow to solidify.
- Incubate the plates of Soyabean Casein Digest Agar at 30-35°C for 3 days and Sabourauds Dextrose Agar at 20-25°C for 5 days.
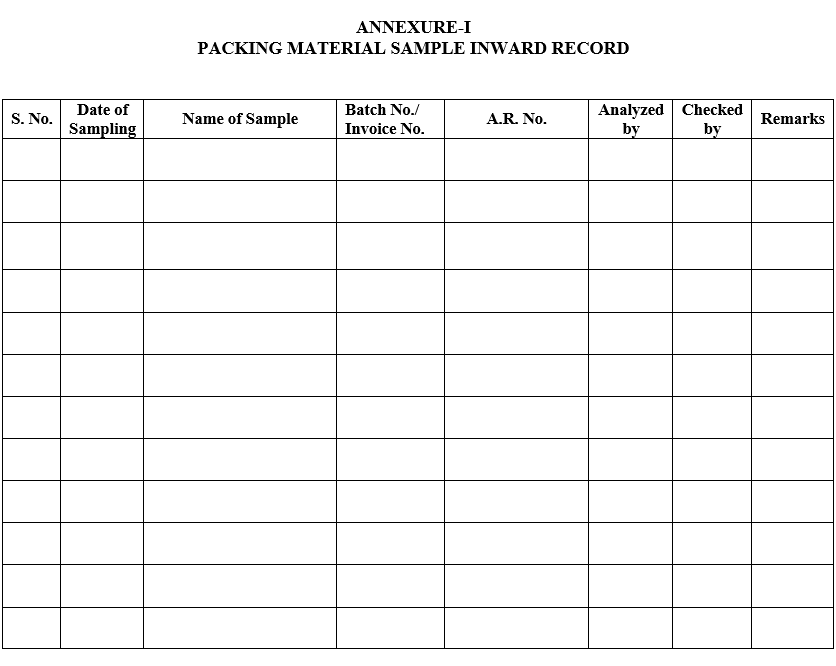
- Microbiologist shall maintain log for inward of Packaging Material as per Annexure I.
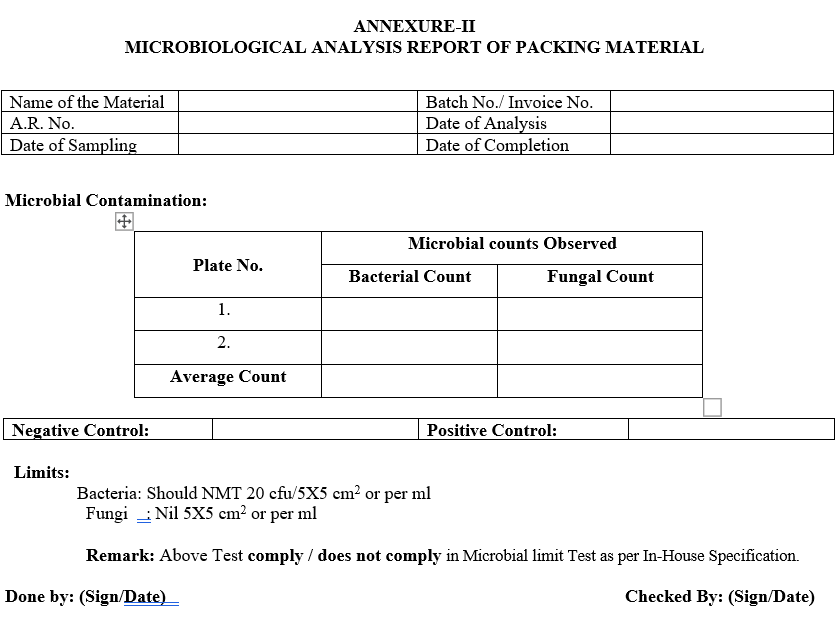
- Count the number of Bacterial and fungal colonies and express the result as count (CFU) / 5×5 cmor per ml as per the Annexure II.
Frequency: Initial three consignments of every new vender and after every three month.
Limits/ Acceptance Criteria:
- Bacteria: Should Not more than 20 CFU/5X5 cm or per ml
- Fungi : Should Not more than 2 CFU/5×5 cm or per ml
- Negative Control: Should not show the growth after 48 Hours.
REFERENCES:
In-House
ANNEXURES:
| ANNEXURE No. | NAME OF ANNEXURE |
| Annexure-I | Packing Material Sample Inward Record. |
| Annexure-II | Microbiological Analysis Report of Packing Material |
DISTRIBUTION:
| Control copy No. 1 | : | Manager Quality Assurance |
| Control copy No. 2 | : | Manager Quality Control |
| Master copy | : | Quality Assurance Department |
ABBREVIATIONS:
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
| MB | : | Microbiology |
| °C | : | Degree Centigrade |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Change | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Recorded Manual |
ANNEXURE-IPACKING MATERIAL SAMPLE INWARD RECORD
ANNEXURE-II
MICROBIOLOGICAL ANALYSIS REPORT OF PACKING MATERIAL