- OBJECTIVE:
- To lay down the procedure for Issue of materials for miscellaneous purpose. (Other than production).
- SCOPE:
- This SOP is applicable for the warehouse department at {Company Name} {Location}.
- RESPONSIBILITY:
- WH Executive/Designee – shall be responsible to follow the procedure as per SOP.
- Head/Designee Quality Assurance is responsible for authorization of internal material requests.
- Head/Designee Warehouse – is responsible for compliance of the SOP.
- ACCOUNTABILITY:
- QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- Issuance of packaging materials for FRD trials and machine trials
- Issuance of processing materials for FRD trials.
- Issuance of cleaning aids like IPA etc.
- Issuance of Raw and Packing Material
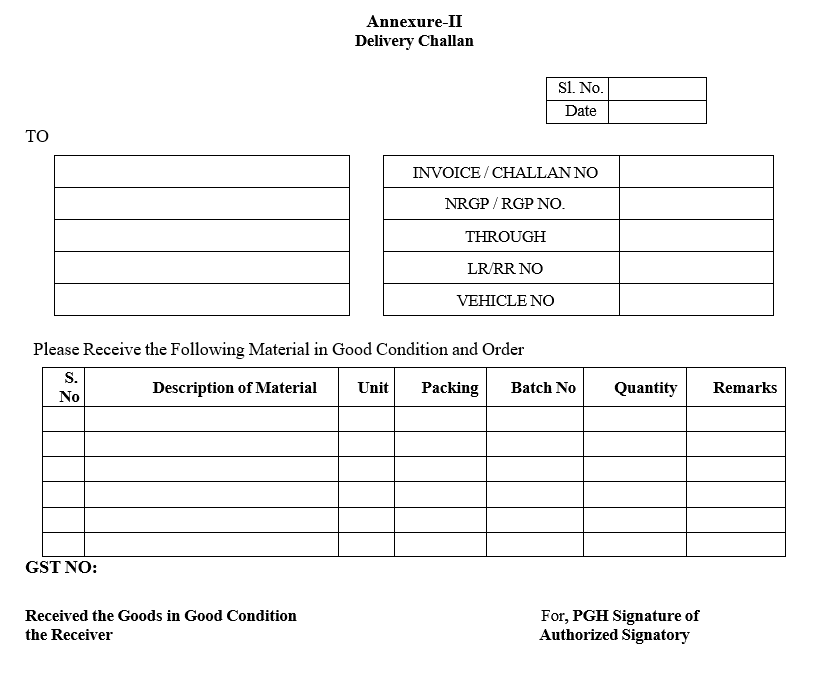
- Initiator department shall raise a Miscellaneous Material Indent Cum Issue Note (Format-I) for the required materials and move order.
Note: All the internal Material requisitions should be authorized by QA in charge
- Based on the Miscellaneous Material indent cum issue Note Warehouse personnel shall dispense and issue the quantities of required material as per the current version of SOP on the FIFO basis.
- If the Miscellaneous Material indent cum issue Note is for the transfer of material to other locations/outside the premises a Delivery challan (Format-II) shall be prepared for transitional purpose and the material shall be dispatched to the respective locations through material entry.
- COA of the concerned material shall be accompanied with the consignment.
- Prepare ‘Material Issue Tag’ through computer and transact the dispensed material in excel sheet. Affix the ‘Material Issue Tag’ to the dispensed material.
- File the Miscellaneous Material indent cum issue Note serially after completion of the dispensing activity.
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Miscellaneous Material Indent Cum Issue Note |
| Annexure-II | Delivery Challan |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Warehouse
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| PGH | : | Pharmaguidehub |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| FIFO | : | First in First out |
| QA | : | Quality Assurance |
| FRD | : | Formulation Research & Development |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
Miscellaneous Material Indent Cum Issue Note
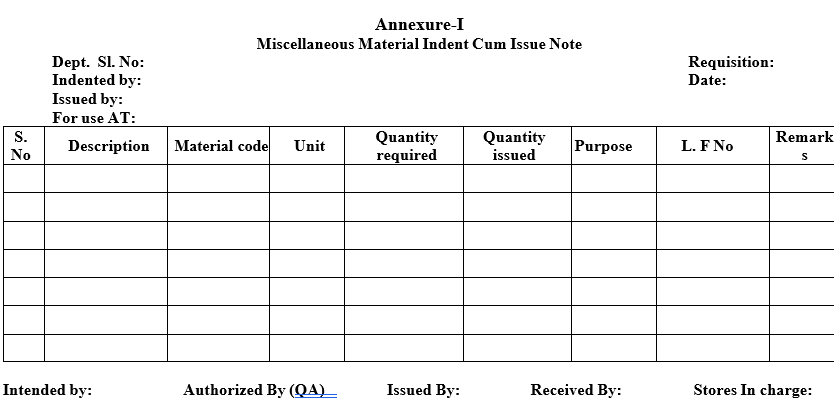
Annexure-II
Delivery Challan