- PROCEDURE FOR PREPARATION, STERILIZATION, STORAGE AND USAGE OF MICROBIOLOGICAL MEDIA:
- Media is a culture media, or growth media. This is a specially prepared gel or liquid that provides nutrients and an environment for microorganisms, like bacteria, to grow in a lab setting.
- Media Preparation:
- Collect approximately 75% of the required volume of purified water into a suitable container.
- Weigh the quantity of dehydrated media for the preparation of agar or broth as per instructions mentioned on the manufacturers label and suspend in the container with purified water.
- Add the remaining 25 % of purified water to make up the required volume.
- Dissolve the powder by gently stirring with glass rod/stirrer, followed by boiling.
- Check the pH of medium and adjust the pH using 1.0 M NaOH or 1 M HCl solution, if necessary.
- If required, dispense the required volume of dissolved medium in desired containers.
- Close the container tightly with a suitable cap for bottles or cotton plug in case of flasks and cover with butter paper sheets and tighten them using cotton thread / rubber bands.
- Label the flask/bottle with medium name, Media reference Number, Date of preparation and use before.
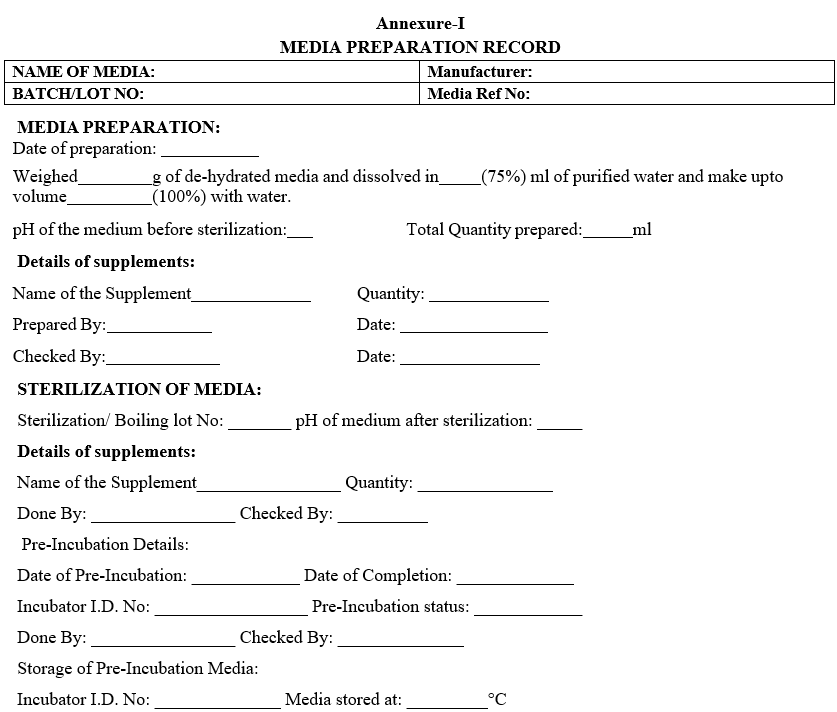
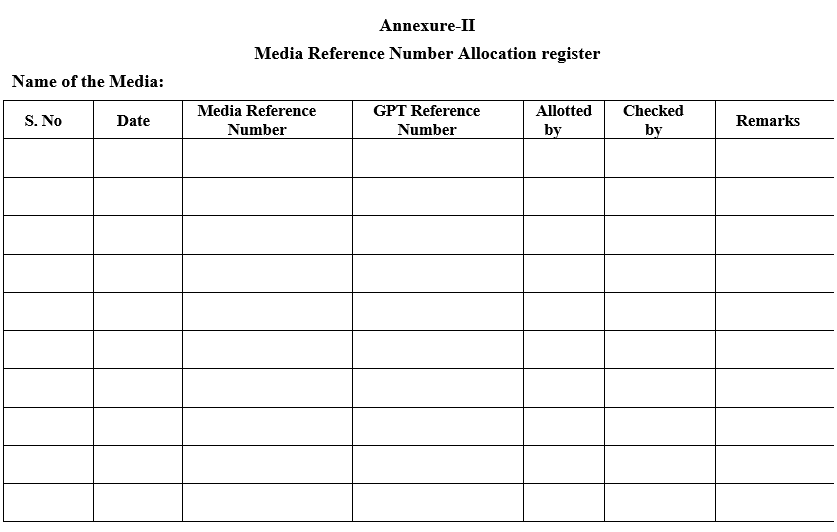
- Record the details in Media preparation record as per Format –I.Assign a Media reference number to the prepared media and enter in Media Reference Number allocation register as per Format-II.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/procedure-for-preparation-sterilization-storage-and-usage-of-microbiological-media/
- Assigning of Reference Number to prepared media:
- All prepared media shall be identified with unique number.
- The numbering system consists of 10 characters.
- The first three characters denote the name of the Media as per the format -III.
- Next character is a “/” slash.
- Next three characters are the serial number starting with 001in every year.
- Next character is a “/” slash.
- Next two characters denote current year.
Example: The first SCD medium in the year of 2025 shall be numbered as SCD/001/25.
- Sterilization of Media:
- All the prepared media shall be subjected for sterilization by boiling or autoclaving as mentioned on the manufacturer Container label.
- Wrap the mouth of the media containers with Kraft / butter paper.
- Affix the autoclave chemical indicator strip on the media containers.
- Sterilization by boiling can be done by using water bath / Hot plate.
- Sterilization by Autoclave can be done as per the SOP.
- After completion of sterilization, ensure the colour of the indicator is changed to black
- If there is no change in colour, discard the material and prepare the media freshly.
- Check the pH of sterilized media and record in Media preparation record. (Format-I)
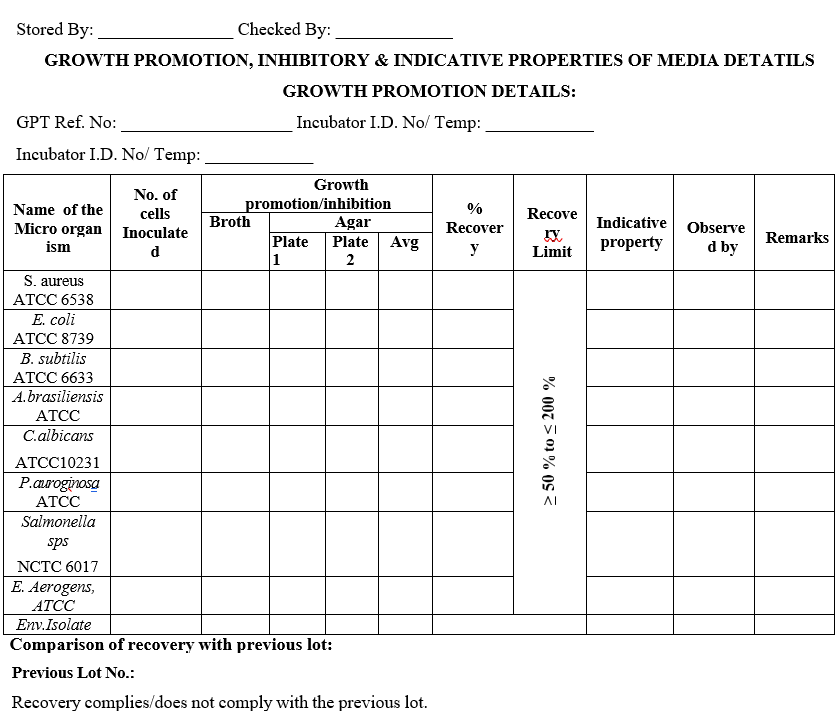
- All the sterilized media shall be subjected to Growth Promotion test as per SOP.
- If the media shows satisfactory growth, then the media shall be used for routine analysis, if not reject the material.
- Record the results in Media preparation record (Format-I)
- Pre Incubation and storage of Pre-Incubated Media:
- All the prepared and sterilized media shall be kept for incubation at 30-35°C up to 48 hours (For Bacterial media) and 20-25°C up to 72 hours (For fungal media).
- After completion of pre-incubation period, visually check the media tubes and plates for the absence of growth, if any contamination observed discard the plates. 5% is the acceptable range for contamination.
- All pre poured plates should be labeled with Name of Media, Media reference Number, Date of preparation and use before date.
- Enter the pre incubation details in Media preparation record. (Format-I).
- Storage and usage of Pre-Incubated Media:
- Transfer the pre-incubated media tubes and plates at 20-25°C Incubator and store up to15 days.
- Take the required pre-incubated media from 20-25°C Incubator and use for Microbiology analysis.
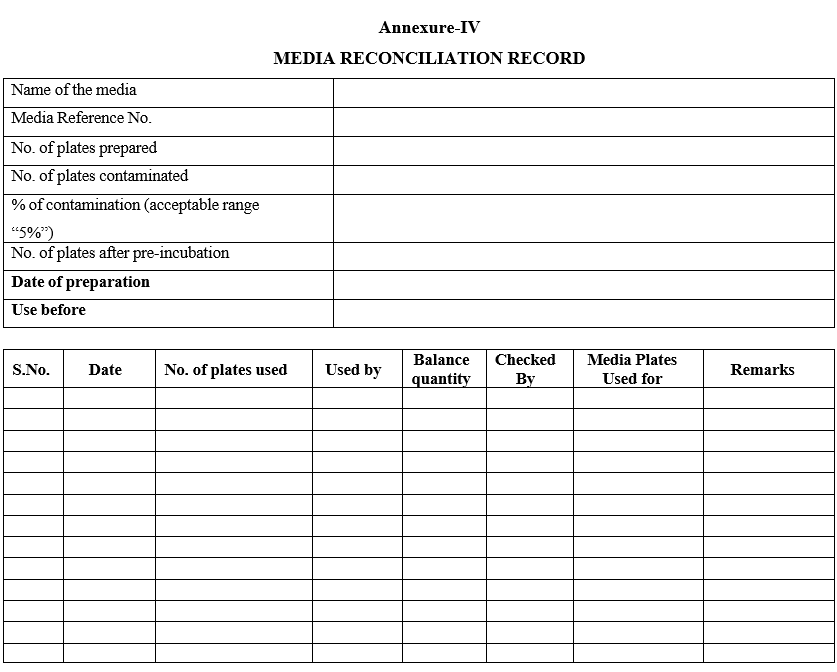
- Enter the details of media usage in media reconciliation record Format IV.
NOTE: Compare the current lot with the previous lot of media for recovery Factor2.
- RecoveryFactor 2 Calculation:
- For minimum value calculation – Number of inoculated cells are divided by 2 E.g.: Number of inoculated colonies – 50/2 = 25 cells. So, the minimum value is 25 cells.
- For maximum value calculation – Number of inoculated cells are multiply with 2 E.g.: Number of inoculated colonies – 50×2 = 100 cells So, maximum value is 100 cells.
- The recovery should between > 50 % to < 200 %.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/procedure-for-preparation-sterilization-storage-and-usage-of-microbiological-media/
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Media preparation record |
| Annexure-II | Media Reference Number allocation record |
| Annexure-III | Media Reference Number allocation record |
| Annexure-IV | Media Reconciliation record |
- ABBREVIATIONS:
| No. | : | Number |
| SCD | : | Soyabean Casein Digest Agar. |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
Annexure-I
MEDIA PREPARATION RECORD

Annexure-II
Media Reference Number Allocation register
Annexure-III
LIST OF MEDIA CODES
| S. No | Name of the Media | Code |
| 01 | Soybean Casein Digest broth | SCB |
| 02 | Peptone | PEP |
| 03 | Soyabean Casein Digest Agar | SCD |
| 04 | Sabouraud Dextrose Agar | SDA |
| 05 | Sabouraud Dextrose Broth | SDB |
| 06 | R2A Agar | R2A |
| 07 | Mac Conkey Broth | MCB |
| 08 | Mac Conkey Agar | MCA |
| 09 | Nutrient broth | NUB |
| 10 | Rappaport Vassiliadis salmonella enrichment broth | RVM |
| 11 | Xylose lysine deoxycholate agar | XLD |
| 12 | Triple sugar Iron Agar | TSI |
| 13 | Mannitol salt Agar | MSA |
| 14 | Cetrimide agar | CEA |
| 15 | Enterobacteria Enrichment Broth | EEB |
| 16 | Violet Red bile Glucose Agar | VRB |
| 17 | Reinforced Clostridial Broth | RCB |
| 18 | Columbia Agar | CBA |
Annexure-IV
MEDIA RECONCILIATION RECORD
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/procedure-for-preparation-sterilization-storage-and-usage-of-microbiological-media/
