- PROCEDURE FOR PROCURING, SUBCULTURING & MAINTENANCE OF CULTURES:
- Procurement of Reference/Mother cultures:
- Reference/Mother cultures shall be procured from an Authenticated National Organizations/International organization like.
- ATCC – American Type Culture collection.
- NCIM – National collection of industrial microorganisms.
- NCTC – National Collection of Type Cultures
- Procure the cultures as and when required as per Format-I. In case of any specific requirement, procure a new culture.
- Ensure that all related compliance certificates are available.
- Receipt & Storage of reference/ Mother cultures:
- After receipt, ensure that the cultures are supplied in acceptance condition and check the physical conditions and enter the details in Format-II.
- Check the Strain name, Strain Number and Passage Number against COA and ensure the correct culture was received.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/procedure-for-procuring-subculturing-maintenance-of-cultures/
- Note: – Reference/Mother culture shall be received with not more than two passages.
- Upon receipt, any form of cultures (Lyophilized ampoules, Vials, Pellets and Culture slants) shall be stored at 2-8°C in original sealed container/ tube.
- Revival of Reference/ Mother cultures:
- Media preparation: Suspend specified amount of Nutrient Agar (NA) or Soya bean casein digest agar (SCD) in required amount of purified water, and adjust the pH, Boil to dissolve and sterilize by autoclaving.
- Preparation of slants: Pour 8ml of media into the test tubes or screw capped bottles and sterilize by autoclaving, allow to cool at an angle of 45° C such that agar forms a slant. For stab culture allow the agar to solidify in upright position.
- Procedure for Revival:
- In case of slants: Reference cultures or Mother Culture obtained as slants are used for sub culturing. Sub culturing is done by taking a loop full of culture from the slant and streaking on the surface of the fresh slant
- In case of lyophilized cultures (ampoules): Tips of the ampoules are heated in the Bunsen flame while still hot add a drop of or two sterile water or sterile liquid media at the tip, to crack the glass. Aseptically remove the tip of the container with the sharp blow with a forceps. Flame the cotton plug tip and aseptically remove the cotton plug. Flame the open end of the vial and proceed for rehydration and sub culturing.
- In case of lyophilized pellets: Take the required pure culture into LAF bench, aseptically open the cup of the culture tube with the help of the sterile forceps, remove the one of the lyophilized pellet form and inoculate into the 50ml sterile Soyabean casein digest broth for bacterial cultures and 50ml sterile Sabouraud dextrose broth for fungal cultures and incubate the bacterial cultures at 30-35 ℃ for 24-48 hrs. and fungal cultures at 20-25 ℃ for 48-72 hrs. respectively.
- Rehydration of lyophilized cultures:
- Aseptically add 0.2ml of sterile SCB with the sterile pipette to the glass ampoules containing the lyophilized cultures.
- Transfer the total culture to the 100ml of SCB.
- Incubate the cells in the medium at 30-35℃ for 24-48 hrs. for bacterial cultures and 20-25℃ for 48-72 hrs for fungal cultures.
Note:-
- Since some cells may exhibit a prolonged lag period before growth, incubation should be continued for at least 2 weeks at the appropriate temperature before discarding the cells as in viable.
- The ampoule should be kept in a refrigerator/Cooling incubator, if it is not opened soon after the receipt.
- The ampoules and remaining contents should be sterilized before discarding.
- Sub culturing:
- Preparation of Primary cultures:
- After achieving the established growth on primary culture slant.
- Label the primary cultures with the respective culture name and store the primary culture at 2-8°C.
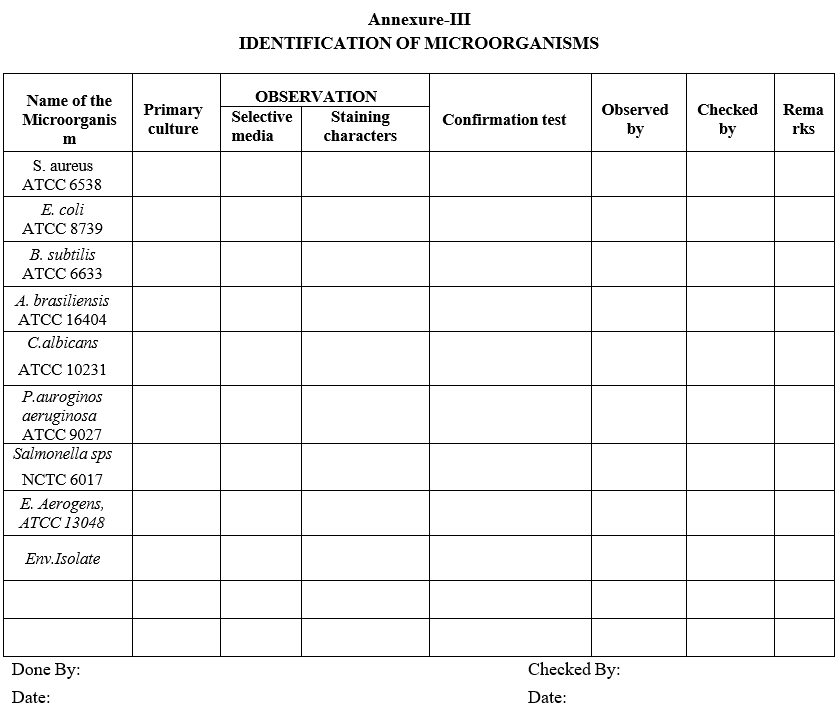
- After achieving the growth on slant, simultaneously perform the gram staining to check the purity of the strain and inoculate on the selective media for identification and conformation of the strain.
- Record the results in Format-III.
- For Bacillus sps and Aspergillus sps perform the gram staining to identification of microorganisms.
- If the purity and identification results are found satisfactory, use the primary culture for further usage.
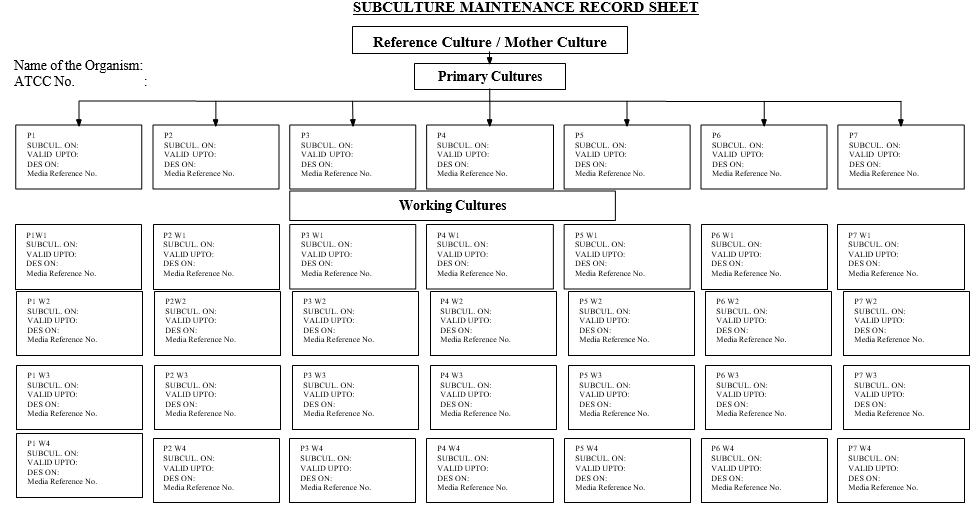
- The primary cultures shall be coded as P1, P2 and P3 ……P7 (6 +1 one culture per month and one culture as standby).
- Stand by culture (P7) shall be used whenever there is requirement.
- Enter the details in Format-IV.
NOTE:- Reference cultures shall not be used more than five passages from the original strain.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/procedure-for-procuring-subculturing-maintenance-of-cultures/
- Preparationof Working cultures:
- Prepare a series of working cultures 5+1 slants from primary culture, depending on the requirements of a particular month.
- To recover the bacteria/ fungi remove the primary culture from the cooling incubator and kept under aseptic conditions for acclimatize to the room temperature.
- Perform the purity check for each primary culture during the generation of working cultures. The working culture shall be coded as W1, W2……W6 prefixed by the primary culture code which it is sub cultured. EX:- P2W1 denotes Working culture-1 sub cultured from Primary culture-2.
- Enter the details in Format-IV.
- Incubate the slants at 30-35°C for 24-48 hrs. for bacterial cultures and 20-25°C for 48- 120 hrs. for fungal cultures.
- All the primary and working cultures shall be labeled as per the following label.

NOTE: – If any contamination observed in any one of the working cultures use the stand by Working culture (W6).
- Preservation of Cultures:
- All the primary cultures and working cultures shall be wrapped with Para film/Aluminum foil and stored at 2-8 °C .
- When not in use, Reference/Mother cultures shall be stored at 2-8 °C till the expiry period.
- The validity period for the primary cultures – Six months.
- Each primary culture shall be stored up to respective month. EX:– P1-Jan, P2-Feb, P3-March, P4-April, P5-May, P6-June and P7- Stand by.
- The validity period for working cultures- One month.
- Each working culture shall be stored up to the respective one week.
NOTE:- If any contamination observed in any one of the primary culture, use the Stand by Primary culture (P7).
- Disposal of Microbial Cultures:
- The Lyophilized microorganisms and subsequent growth on the Media are considered to be biohazard.
- These microorganisms shall be discarded safely.
- Once the revival is completed, decontaminate the original containers at 121°C for 30 minutes.
- Once the Primary cultures are used for the respective month, decontaminate the cultures at 121°C for 30 minutes.
- After usage of working cultures for a particular week, decontaminate at 121°C for 30 Minutes.
- General precautions:
- Since the cultures are pathogenic, they should be handled with great care.
- The persons are trained to handle the cultures inside the LAF.All the cultures shall be discarded prior to the disposal and send it to safety, Health and Environmental (SHE) department for disposal through acknowledgement.
- If any spillage occurred, handle the spillage by flooding with in use disinfectant and further collect the waste and decontaminate prior to disposal.
- If any power failure in between the handling of cultures inside the biological safety cabinet, immediately stop the activity and close the all tubes/bottles.
- After the power resumed, wait for 30 min to stabilize the Laminar air flow.
- After each sub culturing flame the loop till it become red hot.
- Working cultures shall not be stored at 20°C.
- Notify any spillage (Inhalation and any breakage of slants/vials/ampoules) immediately inform to In-charge of Microbiology to take corrective action.
- Disinfect the LAF bench with approved disinfectant before and after activity.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | List of Microbial cultures and maintenance. |
| Annexure-II | Receipt of Microbial cultures |
| Annexure-III | Identification microorganisms |
| Annexure-IV | Subculture maintenance record sheet |
| Annexure-V | Handling of Lyophilized vials/ampoules. |
- ABBREVIATIONS:
| No. | : | Number |
| SCD | : | Soyabean Casein Digest Agar. |
| NCIM | : | National Collection of Industrial Micro Organisms. |
| LAF | : | Laminar Air Flow |
| SHE | : | Safety, Health and Environmental |
| IPA | : | Isopropyl alcohol. |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/procedure-for-procuring-subculturing-maintenance-of-cultures/
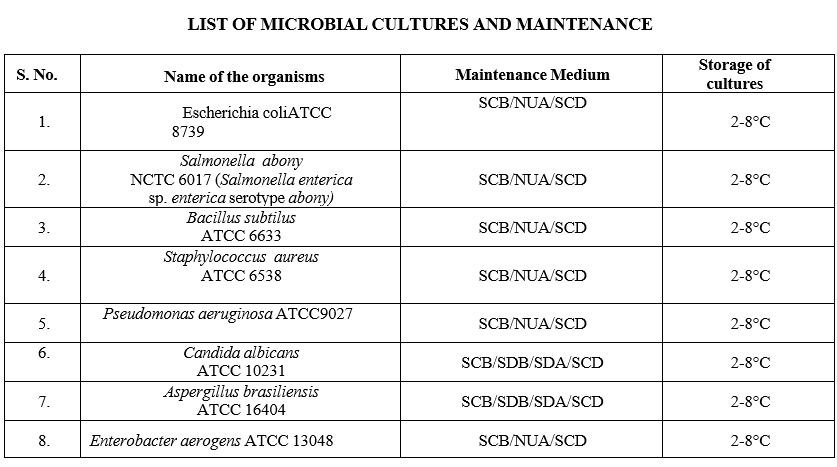
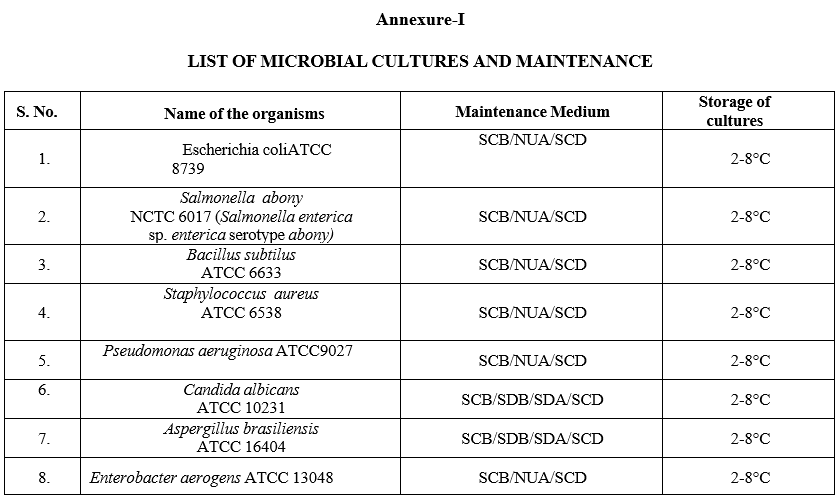
Annexure-I
LIST OF MICROBIAL CULTURES AND MAINTENANCE
Annexure-II
RECEIPT OF MICROBIAL CULTURES
| S. NO. | NAME OF THE ORGANISMS | Lot No. | Received by/Date | Valid up to | Storage Condition | Remarks |
| 1. | Escherichia coli ATCC 8739 | |||||
| 2. | Salmonella abony NCTC 6017 (Salmonella enterica ssp. enterica serotype abony) | |||||
| 3. | Bacillus subtilis ATCC 6633 | |||||
| 4. | Staphylococcus aureus ATCC 6538 | |||||
| 5. | Pseudomonas aeruginosa ATCC 9027 | |||||
| 6. | Candida albicans ATCC 10231 | |||||
| 7. | Aspergillus brasiliensis ATCC 16404 | |||||
| 8. | Enterobacter aerogens ATCC 13048 |
Annexure-III
IDENTIFICATION OF MICROORGANISMS
Annexure-IV
Subculture Maintenance Record Sheet
Annexure-V
HANDLING OF LYOPHILIZED VIALS/AMPOULES
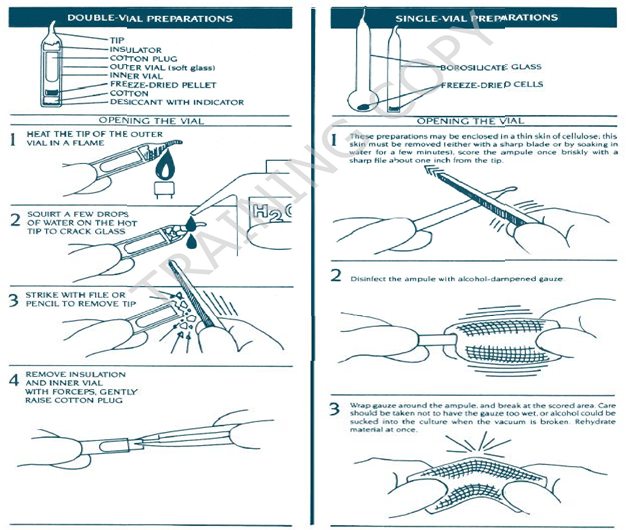
Note: Revive the lyophilized Pellets and Slants as per Manufacturer/Supplier instructions.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/procedure-for-procuring-subculturing-maintenance-of-cultures/

