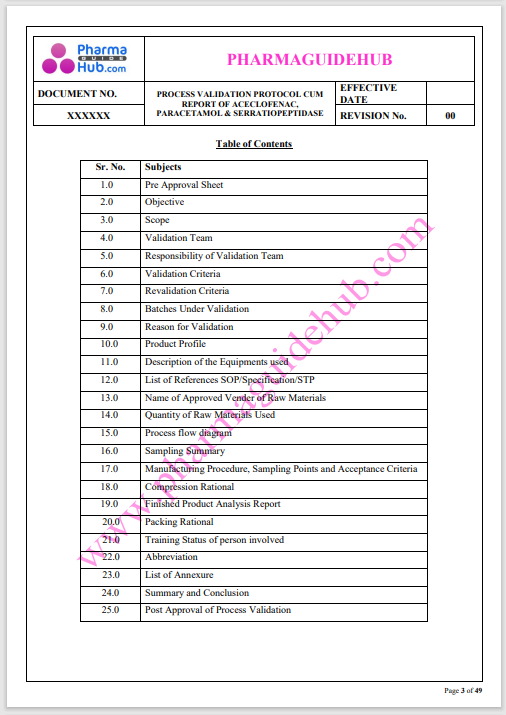
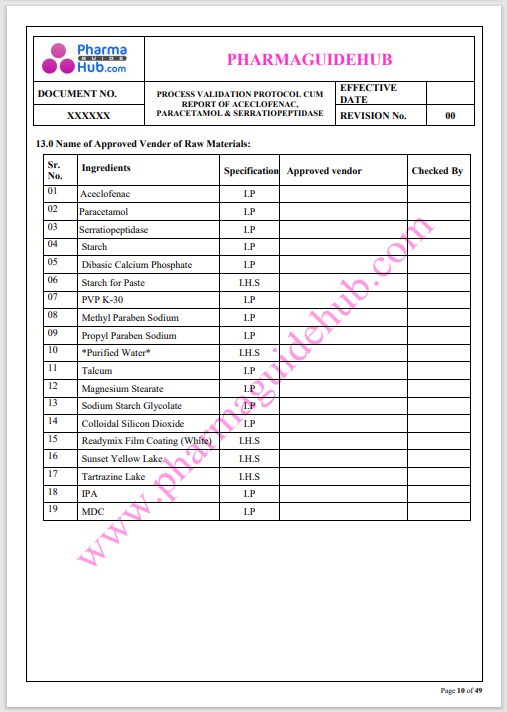
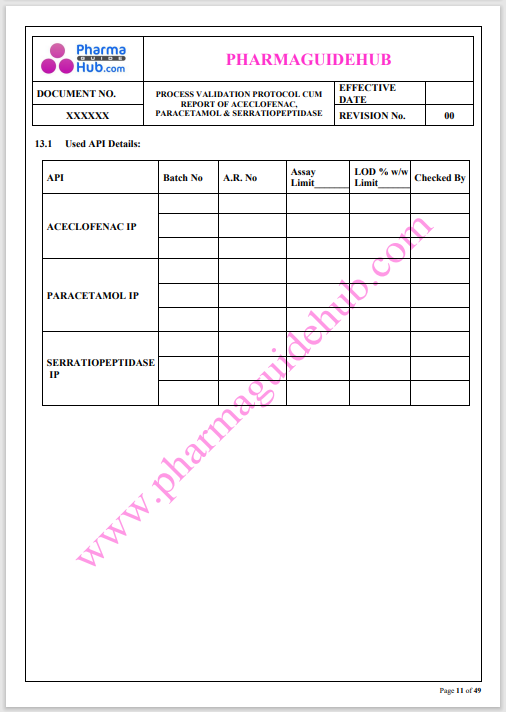
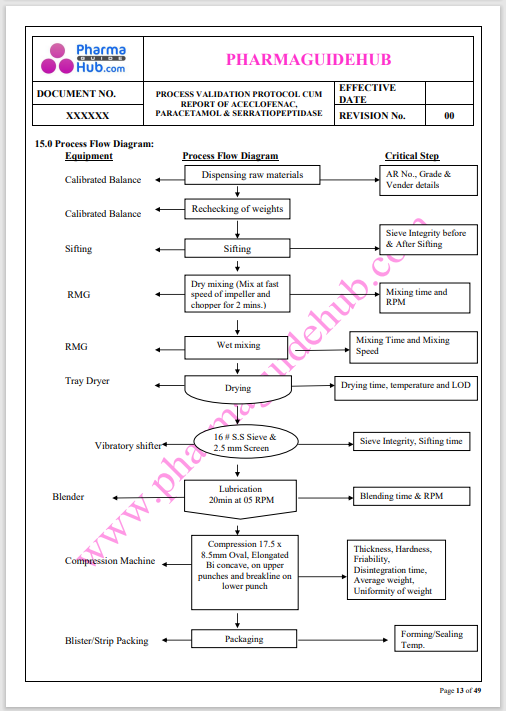
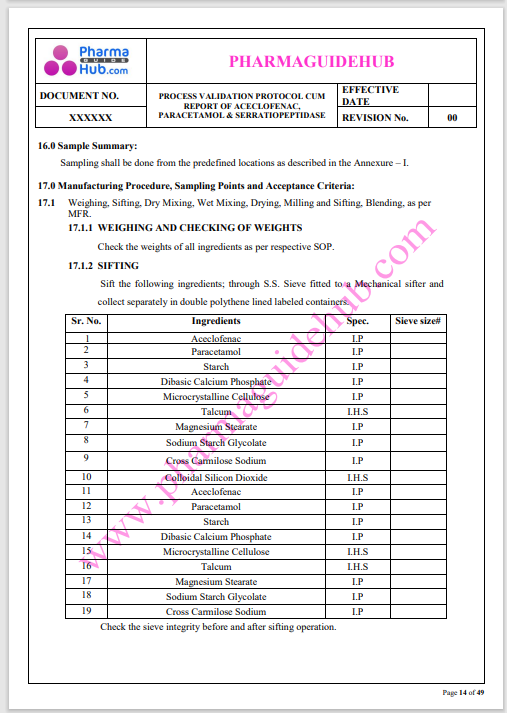
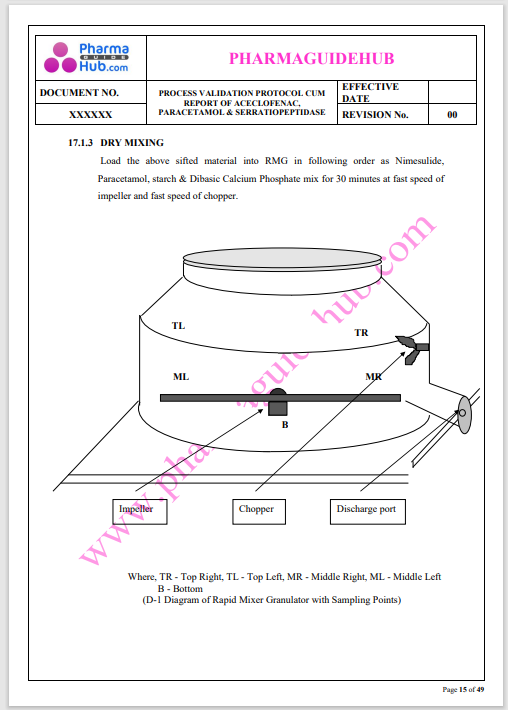
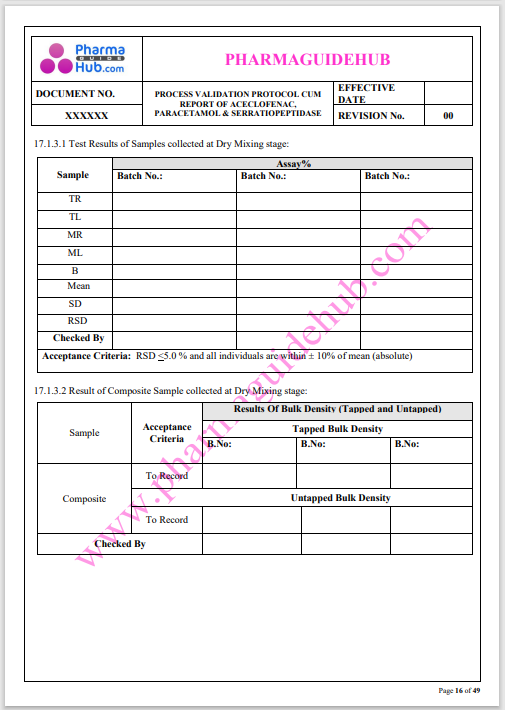
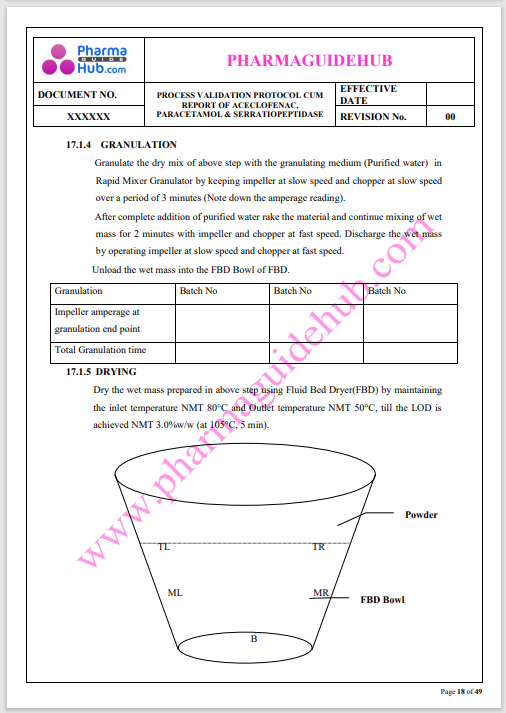
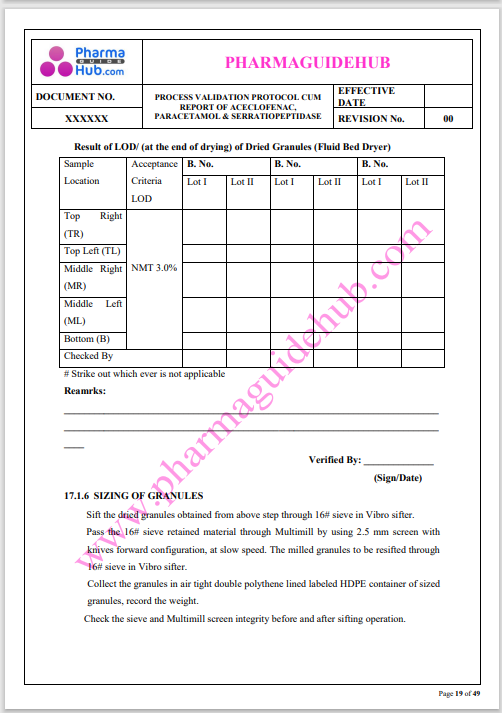
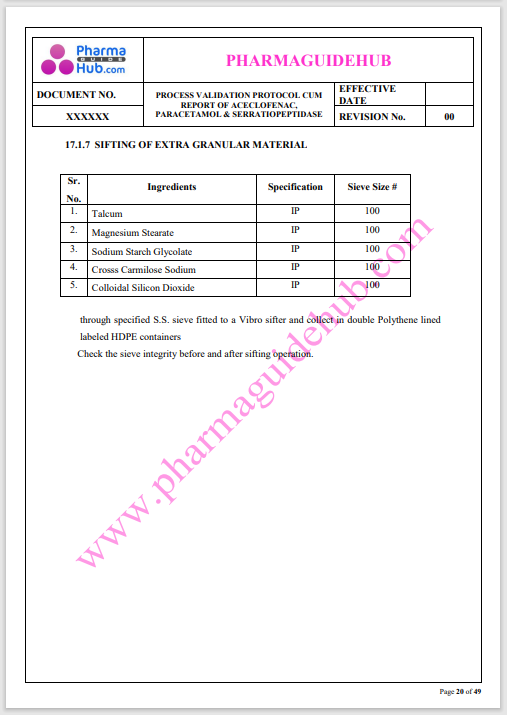
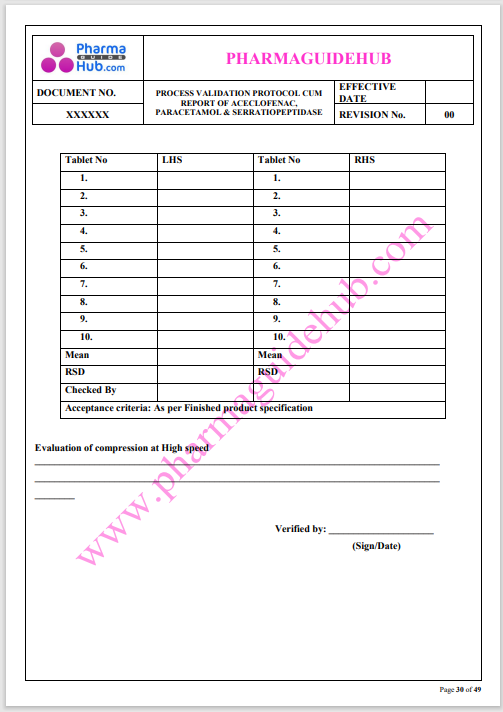
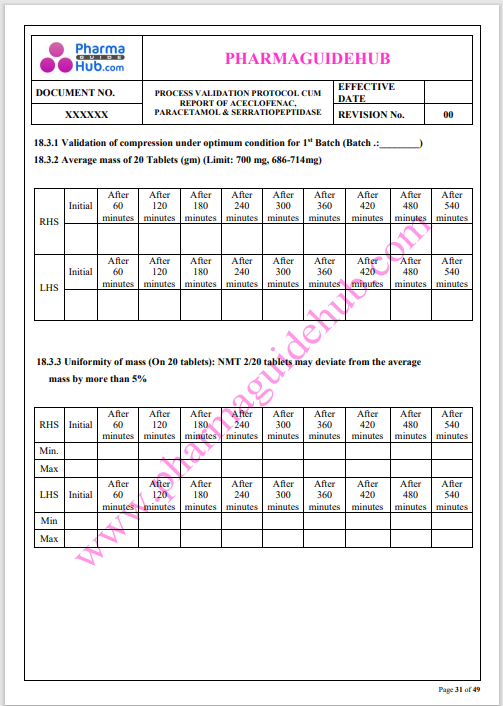
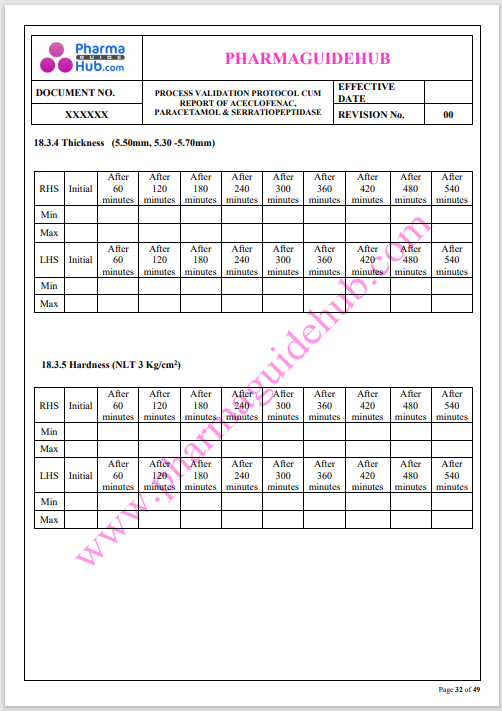
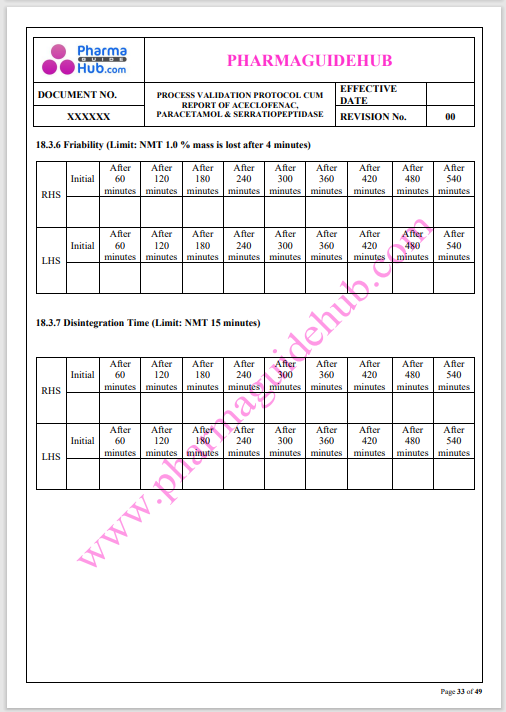
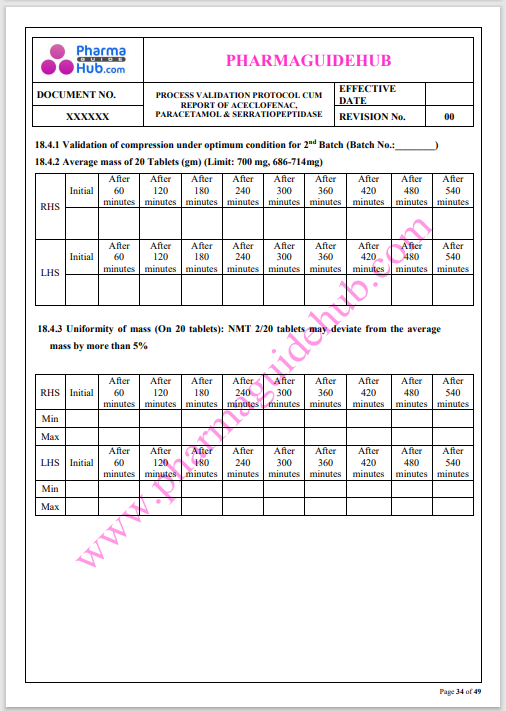
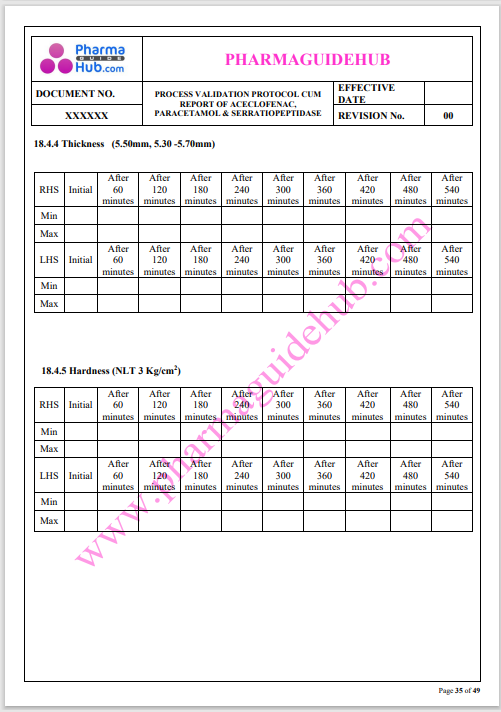
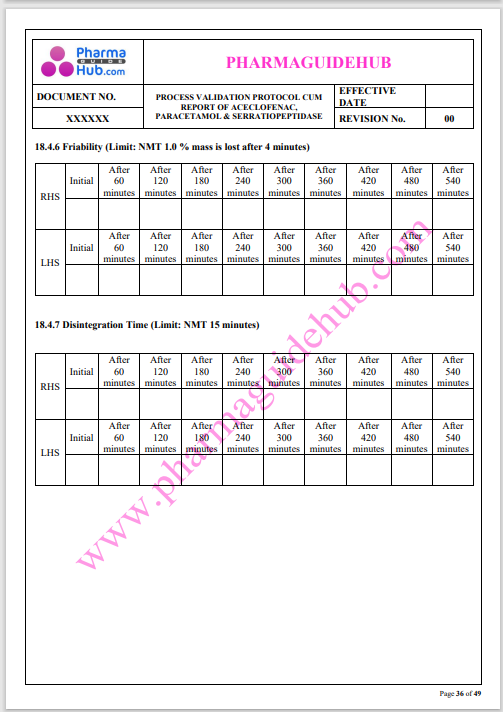
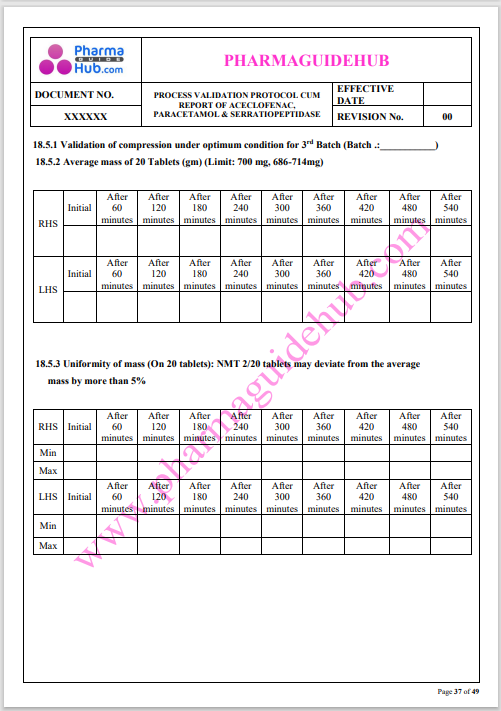
Process Validation: Process validation is established documented evidence that provides a high degree of assurance that a specific process will consistently produce a product, meeting its predetermined specifications and quality characteristics.
This document contains the detailed process validation details of Aceclofenac, Paracetamol & Serratiopeptidase tablets.