- OBJECTIVE:
To lay down a procedure defining an action plan for recall/ withdrawal of product from use or supply, or subject to corrective action, for reasons relating to their quality, safety, or efficacy.
- SCOPE:
This procedure is applicable to all the goods returned from the market at {Company Name} {Company Location}.
- RESPONSIBILITY:
- HEAD QA:
- To decide, monitor and ensure effective recall of product.
- To communicate with party regarding recall.
- To inform regulatory authorities immediately after recall decision is taken in case of serious adverse pharmaceutical reaction (APR) and adverse event.
- Informing the management about any product defect which may require recall.
- Overseeing the system for receiving, verification, quarantining, segregation and securing of recalled stock.
- To initiate and lead the investigation process.
- Making decision on disposition of recalled stock as per this SOP.
- To review the current and previous manufactured batches of product
- PRODUCTION HEAD:
Provide technical support during investigation.
- WAREHOUSE HEAD:
- Receipt, verification, quarantine/ segregation, and secured storage of recalled stock.
- Informing QA about the recalled goods.
- Providing support during recall investigation.
- HEAD QC:
- To analyze the samples of recalled products.
- To provide the analytical report for the recalled product.
- To assist QA in the investigation process of the recalled products.
- DISTRIBUTOR/ MARKETING AUTHORIZATION HOLDER:
- To ensure the removal of impugned batch of product from the market in a stipulated time as per class of the recall.
- The marketing head acts as chief coordinator to collect information on recall/withdrawn to and from the key points in the distribution chain. He shall be company’s spokes-person in matters related to publicity / communications, where the need arises.
- In the case of report of any adverse drug reaction, QA Head shall contact for prompt action and further investigation.
- ACCOUNTABILITY:
QA Head shall be accountable for implementation of this SOP.
- PROCEDURE:
- RECALL / WITHDRAWAL:
- WITHDRAWAL:
- A productmay be withdrawn from sale for two reasons:
- The product has a quality defect or is underweight or has labeling irregularities that does not pose a potential risk to public health and safety.
- As a precaution, stock may be withdrawn from distribution pending further investigation, if a risk to public health and safety is established, the product must be recalled.
- Withdrawals to be informed to the concerned Regulatory Authorities.
- Withdrawals do not require notification to media.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/product-recall/
- RECALL:
Recall is defined as ‘action taken to remove the product from sale, distribution and consumption which deficiencies are reported in quality, efficacy or safety. The defective product related to quality includes Not of Standard Quality, Adulterated or Spurious drugs. Safety and efficacy related recalls include serious adverse reactions and death.
- There could be two alternative actions:
- Permanent removal of unsafe products from the market or from use.
- Temporary removal of the unsafe products from the market, followed by rectification of the problem and a return to the market.
- Product recall also includes drugs prohibited under the provision of Drug & Cosmetic act and also for those products for which product licenses are suspended/cancelled.
- Recall shall be done as per guideline/procedure of the Marketing Authorization Holder provided in the Agreement with (Company name) and the procedure as listed further.
- CLASSIFICATION OF RECALLS:
- Class I is a situation in which there is a reasonable probability that the use of, or exposure to, a defective product shall cause serious adverse health consequences or death.
Example:
- Wrong product label (Labels and content are different product)
- Correct product but wrong strength, with serious medical consequences.
- Chemical contamination with serious medical consequences.
- Mix-up of some product (rogues) with more than one container involved.
- Wrong active ingredient in multi-component product with serious medical consequences.
- Class II is a situation in which the use of, or exposure to, a defective product may cause temporary adverse health consequences or where the probability of serious adverse health consequences is remote.
Example:
- Miss labelling e.g. wrong or missing text or figures.
- Missing or incorrect information- leaflets or inserts.
- Chemical / physical contamination (significant impurities, cross contamination, particulates).
- Class III is a situation in which the use of, or exposure to, a defective product is not likely to cause any adverse health consequences.
- TYPE OF RECALLS:
- Voluntary Recall : Voluntary recall can be triggered by any incident that affects the quality, safety and efficacy of the batch/product in question such as:
- If the batch or batches are found to be not complying with the regulatory specifications during the post marketing stability study.
- If the batch is found to be defective during investigation of market complaint.
- During any failure investigation, if it is observed that the failure under investigation might have adverse quality impact on already released batch (e.g. possibility of contamination, mix-up, degradation etc.)
- If any unusual observation is noted during visual inspection of retention samples, which indicate an impact on quality of the product after investigation.
- If the post marketing surveillance reports /Pharmacovigilance reports indicates that there is serious safety risk associated with the product.
- Statutory Recall: Statutory recall can be triggered in response to the direction or mandate by the drug regulatory authorities in one or more of the situations as follows:
- To recall the drug product/batch, considered to be in violation of the laws, it administers such as not of standard quality.
- To recall the drug product/batch, consider the regulatory/Statutory notification.
- To recall the banned product.
- Labeling and / or Promotional materials that are considered to be in violation of law.
- In the event of identified discrepancy in product, recall shall be called by Head QA and party shall be informed as per Annexure-I.
- Timelines for Effective Recall System: Based on the category of risks involved, the timeline for effective recall system is followed as per Marketing Authorization Holder / Client System Procedure.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/product-recall/
- PRE-RECALL INVESTIGATIONS:
- All communications from the drug Regulatory Authorities, in matters related to product quality has to be expeditiously sent to Head QA and RA, who shall register the same and forward it for immediate evaluation and actions.
- The receiptof the communication/complaint has to be appropriately acknowledged by QA within 24 hours of receiving the communication / complaint.
- Investigationswithout the ‘complaint’ sample at hand shall be considered as of limited value and shall be generally restricted to only checks of the relevant documentation, and ‘reserve samples’ from our own depository. Unless there is strong positive evidence, such investigation cannot be normally considered adequate to recommend extreme actions of product ‘Recall’.
- In-houseinvestigations (including analysis of complaint and the reserve sample) shall be completed within seven working days. Delay (if any) must be justified.
- Additional/ extended investigation shall be carried out for pre and post batches of the same product or other products which may become suspect. In case of extended / additional investigation, the time frame for investigation may be extended and shall be justified for extension of investigation. However, if the quality defect is considered serious enough to endanger the safety of the user of the product, immediate action to STOP SALE shall be taken by Head QA.
- If the investigations indicate a reasonable degree of certainty that the continued presence of materials in the distribution chain and thus their availability to the users is potentially harmful to the users, a ‘Recall’ shall be immediately affected.
- In-houseinvestigation shall be conducted as detailed above even when the ‘Recall’ has been directed/ordered by a drug Regulatory Agency. (It is understood that under this situation the recall is no longer ‘voluntary’. However, the recall approving authority must still approve it).
- RECALL ACTIVITIES:
- CONDUCTING A PRODUCT RECALL:
- There are three primary objectives for any Product Recall:
- Stop the distribution and sale of the affected product.
- Inform the statutory authorities and the public (consumer recalls only).
- Effectively and efficiently remove the product from the market place, which is potentially unsafe.
- The key steps in conducting a successful recall are:
- Hazard / risk assessment.
- Determining the level of the recall.
- Determining who should be notified for the recall.
- Determining the mechanics of notification and recovery.
- Post recalls reporting.
- Hazard / Risk Assessment:
For a product recall, it shall be required that all necessary information is obtained and analysed before a decision is made to initiate a recall. The decision is made by QA.
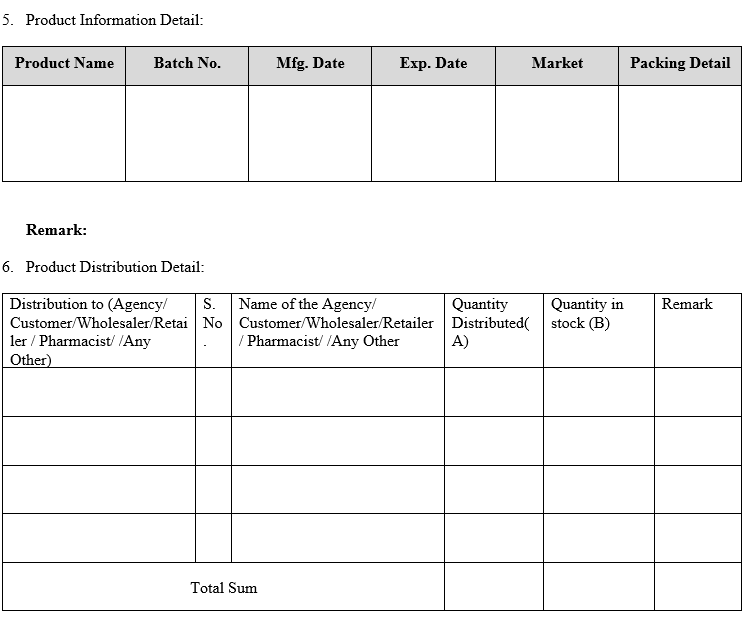
The following information is required for assessment:
- The Problem:
- Nature of the problem (type of hazard and assessment of risk).
- Results of tests and other investigations on suspect sample or other samples.
- The Product:
- Product name, batch number, packs description, including package size and type.
- Manufacturing date, Expiry date.
- Distributor / whole seller contact telephone number (including after hours number).
- Quantity of the batch, date and quantity released.
- Distribution list.
- Other relevant information:
- Name and telephone number of the person who has reported the problem.
- Date of the problem.
- Number of similar reports received (e.g. customer complaints).
- Availability for investigation of suspect samples or other samples.
- Action proposed.
- Proposed recall level.
- Level of Product Recall:
The level (or depth) of recall of a product/batch shall be determined based on recall classification and level to which distribution has taken place.
There are three levels of recall such as consumer/user, retail, and wholesale.
- Consumer or User Level: This may vary with product, including any intermediate wholesale or retail level. Consumers or users may include individual consumers, patients, physicians, and hospitals. All Class I recall shall be executed to the levels of Wholesale/Distributors, retail, and consumer. In such cases, public announcements shall be made using print/electronic media aids viz. Newspapers, Television, Radio etc.
- Retail Level: Recall to the level immediately preceding consumer or user level. It includes retail groceries, pharmacies, hospital pharmacies, dispensing physician, institutions such as clinics and nursing homes, etc. All Class II recalls shall be executed up to the levels of wholesale and retail.
- Wholesale Level: All distribution levels between the manufacturer and retailer. All Class III recalls shall be executed up to the levels of wholesale.
- Notification for the Recall:
Notification has three aspects.
- Notifying statutory authorities and Marketing Authorization Holder (customer).
- Notifying the distribution network/chain and trade customers.
- Notifying the public (in case of a consumer level recall).
- Post Recall Reporting:
- A recall plan should include a recording system for logging product that has been returned in order to ensure that all product is retrieved .The effectiveness of a recall is assessed on the basis of the amount of product received as a proportion of the amount of product that left the manufacturing site, while taking into account the retail turnover of the product.
- In addition to assessing the effectiveness of a recall, it is necessary to follow up by investigating the reason for the recall and taking action to prevent a recurrence of the problem.
- The decision on recall of the defective product/batch shall be made within 24 Hours for Class I recall upon receipt of the intimation.
- Marketing Authorization Holder/Customer shall be informed about the action/decision.
- Within 24 hours of the decision taken for the recall of the product/batch (es) the communication shall be sent by party stating the severity of the defect, using the fastest mode of communication which may include email, telephone, fax, SMS etc. to the entire supply chain.
- The party shall inform the concerned regulatory authorities where the product batch in question was distributed immediately after the decision of recall has been taken. Further actions on recall shall be undertaken according to class of recall.
- Product recall log shall be maintained by QA as per Annexure-II.
- Once the ‘Recall’ has been authorized a clear distinction is made between critical (Class I), major (Class II) and minor (Class III) defects.
- All stocks of the recalled products at Warehouse shall be identified as “Recalled stocks, not to be sold” and stored in the product recall room under lock and key while awaiting a decision.
- Product recall shall be recorded as per Annexure-II.
- Material returned under recall shall be disposed & documented as per Annexure-II.
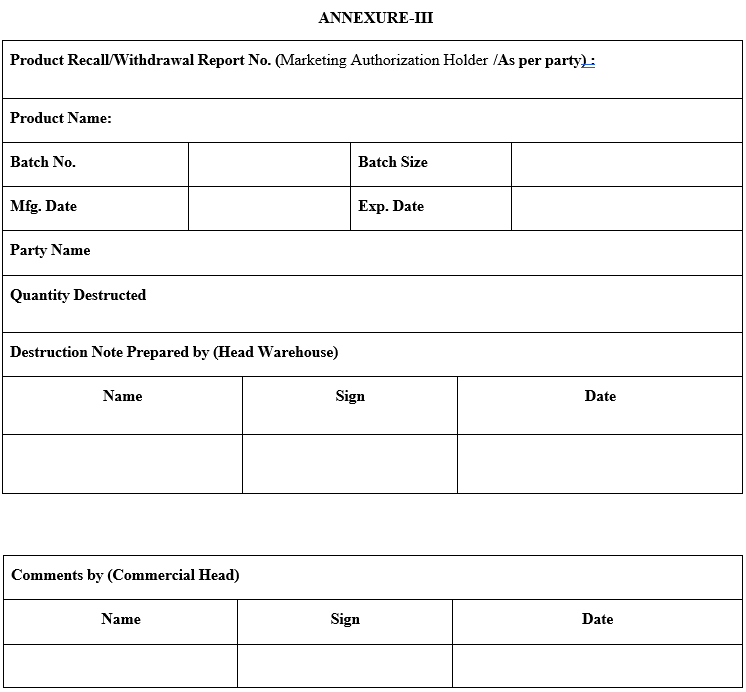
- Recalled Material Destruction Note shall be filled as per Annexure-III.
- Where situation warrants (Life Threatening causes) public announcement shall be made through: press, radio, TV, special bulletins, hand bills, to reach retail outlets, hospitals, medical practitioners & even the individual users.
- The process of collecting the stocks under question shall be completed within a period of 1 Month. The product causing life threats shall be recalled within 10 days.
- Expert advice may be needed to determine the seriousness of the hazard. The team investigating should also consider the possibility of the same problem or type of contamination occurring in different package sizes of the same line, in product with a different batch or in a different product line all together or same or similar product packaged at the same time.
- If the hazard /risk are found to be because of one or more raw materials supplied, then the supplier of raw material and purchase department need to be notified and alerted. Early and comprehensive notification to the distributors will result in a speedy and efficient recall.
- All suspect stocks, including unsold / not distributed / unused stocks along with free samples (if any) shall be received at designated places in the specified areas, and shall be quarantined there and conspicuously labeled, “RECALLED STOCKS. NOT TO BE SOLD”.
- The warehouse department shall keep the co-ordination with customer (party) regularly and completely informed of the process. The progress of the recall process shall be recorded, and final report issued, including reconciliation between the delivered and recovered quantities of the product.
- Head Warehouse shall perform complete reconciliation of the delivered and recovered quantities of the product in a “Summary Report of recall/withdrawal” and forward to Head QA and party.
- Head QC shall analyze the sample of recalled product and provide the analytical report for the recalled product.
- The warehouse head shall keep the record and inform the approving authority (Head QA).
- Head QA shall decide the most effective & safe method for reprocess/ disposing/ destroying the recalled stocks, including packaging materials.
- Head QA shall authorize the reprocess/disposal/destruction of the recalled stocks as per the relevant SOP.
- The disposal/destruction shall be carried out at site(s) approved by Head QA under supervision of QA officer.
- QA department shall prepare the disposal/destruction record of the recalled stocks giving details of product(s) name (including brand name, if any), Batch No., manufacturing & expiry date as given on product, quantity destroyed and signed by the officer/executive under whose supervision the operation was done.
- Head QA in Co-ordination with warehouse head shall prepare a full report detailing:
- Reason for Recall / Freeze.
- Results of full investigation into the cause(s) of the defective product.
- Action taken to Recall.
- Numerical details of the reconciliation exercise.
- Action taken to prevent re-occurrence.
- If the recall is to the consumer level, advertisements paid for by the manufacturer are to be placed in the daily print media of each State in which the product may have been distributed.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/product-recall/
- CHOICE OF PRINT MEDIA:
- The choice of print media should be made in consultation with the QA and the Marketing Authorization Holder/Customer.
- The text of the recall advertisement shall be submitted to the QA and Marketing Authorization Holder /Customer (for confirmation) before it is sent for publication.
- It is important that, whenever possible recall advisements appear on the front pages of daily print media.
- If this is not possible, they should appear in the first half of the newspaper.
- The advertisements should have a heading “Product Recall”.
- DISTRIBUTION RECORDS:
- Distribution records are an important part of the recall product.
- It is therefore strongly recommended that the Marketing Authorization Holder customer is able to produce up-to-date lists of distributors and retailers when needed and the same has to be stated in the agreement also.
- Product recoveries:
- Product may be recovered by returns via distribution chains or direct returns from consumers. The recovered product should be returned to Warehouse of {Company Name} The recovered product must be stored in an area that is separated from any product.
- Accurate records must be kept on the account of the amount of recovered product and batch identification details of that product.
- After recovery, a product may be corrected so that it is fit for human consumption. Corrective action should only be conducted after full consultation with the QA and concern health authority.
- If it is unfit for human consumption and is stored in an isolated country area it may be destroyed or denatured under the supervision of the local health authority.
- If the product is stored in a metropolitan area, it should be destroyed or denatured as directed by the State or territory health authority.
- DISPOSAL OF NON-RETURNED PRODUCT:
- Marketing Authorization Holder/Customer must discuss the disposal of the non returned product with Head QA for product disposal.
- If Head QA decides that the return of distributed product to the company is not feasible, it is the Customer (party) responsibility to instruct customers in the course of notification as to how to safely dispose the product so that it cannot be used for human consumption.
- This information is to be recorded on the recall distribution register so that it can be used to access the overall effectiveness of the recall.
- ACCEPTANCE OF THE RECALL:
- Class I- 100 % of total number of consignee contacted.
- Class II- At least 60% of total number of consignee contacted. However, the percentage may vary on case to case basis.
- Class III – 10 % of the total number of consignee contacted.
- IMPACT ASSESSMENT:
- The impact based on the cause identified for the product recall shall be extended to pre and post batches of the same product or other products/batches manufactured. In case it is established that there is risk involved in such products / batches, the same shall be considered for recall. The impact assessment shall cover the risk on the quality of the products.
- The impact assessment shall cover the risk on the quality of the products manufactured. Also the CAPA instituted will be evaluated for implementation as part of GMP enhancement.
- The impact assessment shall be extended to the vendors of Raw and Packing materials for any preventive actions to be taken at the vendors end in case it is established that the recall has been due to the quality of the input materials and the material in the supply chain may pose a risk on the quality of the future batches manufactured. This may call for auditing the vendor’s manufacturing site to find the cause.
- TERMINATION/COMPLETION OF PRODUCT RECALL:
- A recall shall be terminated when it is confirmed that the batch subject to recall has been removed from sale and necessary disposition has been done.
- Head QA shall finally review the complete recall operation, including all documentation & close the recall as “RECALL COMPLETED”.
- The Product Recall procedure shall be regularly reviewed for their effectiveness and corrective actions taken if deficiencies are observed in the system.
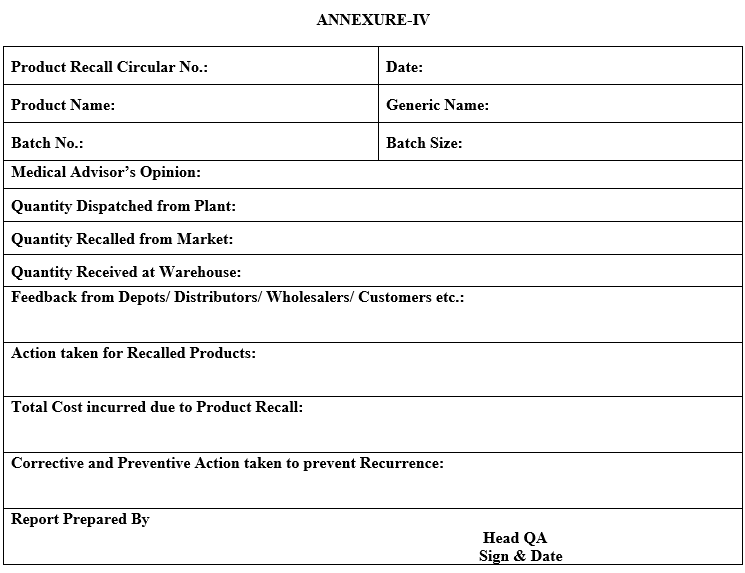
- After completion of all activity for Product Recall, QA Head shall prepare a summary report as per Annexure-IV and same shall be circulated to the all concerned departments.
- Mock recall :
- A Mock recall procedure shall be conducted once in a year & report for the same shall be maintained by QA.
- Mock recall is limited only up to the level of collecting the product distribution information in Coordination with recall committee with a specified time frame.
- Planner for Mock recall shall be prepared as per format as shown in format Mock Recall. Additional mock recall due to any reason shall be record in same.
- For Mock Recall distribution detail shall be filled as per format Distribution Details.
- Mock Product Recall Report shall be prepared as per format Mock Product Recall.
- Information of the quantity of Batches laying the distribution chain shall be collected by QA/PPIC team for making effective product recall.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/product-recall/
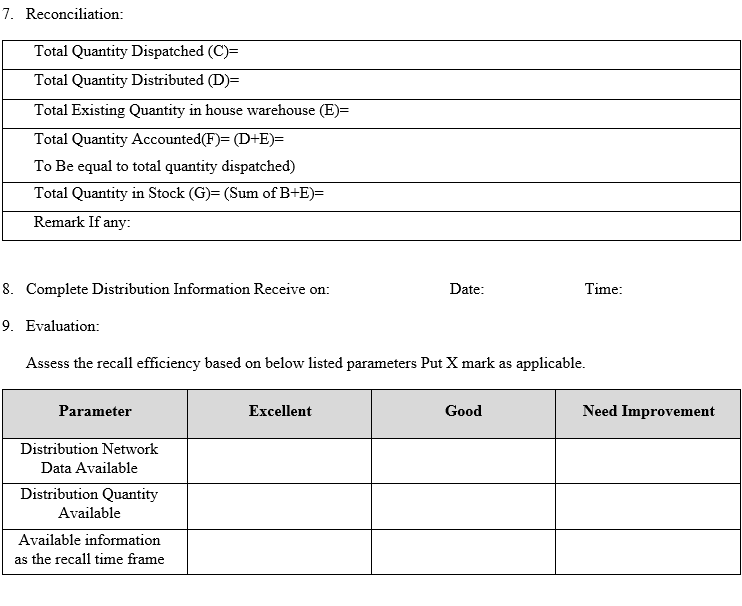
- Rating of Evaluation Parameter:
| Parameter | Excellent | Good | Need Improvement |
| Distribution Network Data Available | 0-12 Hrs | 12-24 Hrs | More than 24 Hrs |
| Distribution Quantity Available | 0-12 Hrs | 12-24 Hrs | More than 24 Hrs |
| Available information as the recall time frame | 0-12 Hrs | 12-24 Hrs | More than 24 Hrs |
- Tracking Forward and Back Recall:
- For traceability, parts and products are identified individually or by lots and information is accumulated in each process. Tracing forward means using accumulated information to track the movement of products and tracing back means tracking records (BMR/ BPR/ Material Movement in individual batch) backward in the timeline.
- Tracing forward is an action to track a product by following the timeline. When a defect is detected in a particular part, batch or lot for instance, products containing the part can be identified to recall them precisely. Consequently, it is effective for the measures against recalls and defective products.
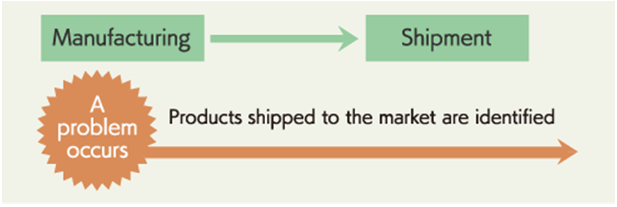
- Tracking backward is an action to track records backward in the timeline. For example, when a problem occurs with shipped products, the relevant lot and process (Raw material, packing material detail) can be identified to investigate the cause promptly. Identification of a lot or a process allows quick actions to improve processes and quality, which leads to higher and more stable product quality.
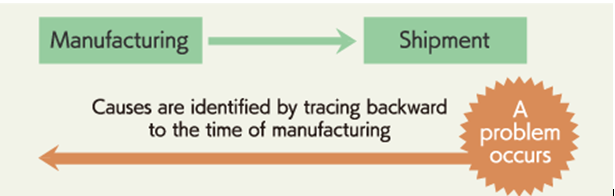
- For tracking backward investigation shall be carried out and during investigation manufacturing process, equipment, utility, man, material, method and mother nature shall be consider and based on investigation needed corrective and preventive action shall be taken. As per customer requirement investigation report shall be sent.
- Recall to be done as per international guidelines and Global Pharmaceutical Regulatory Authorities.
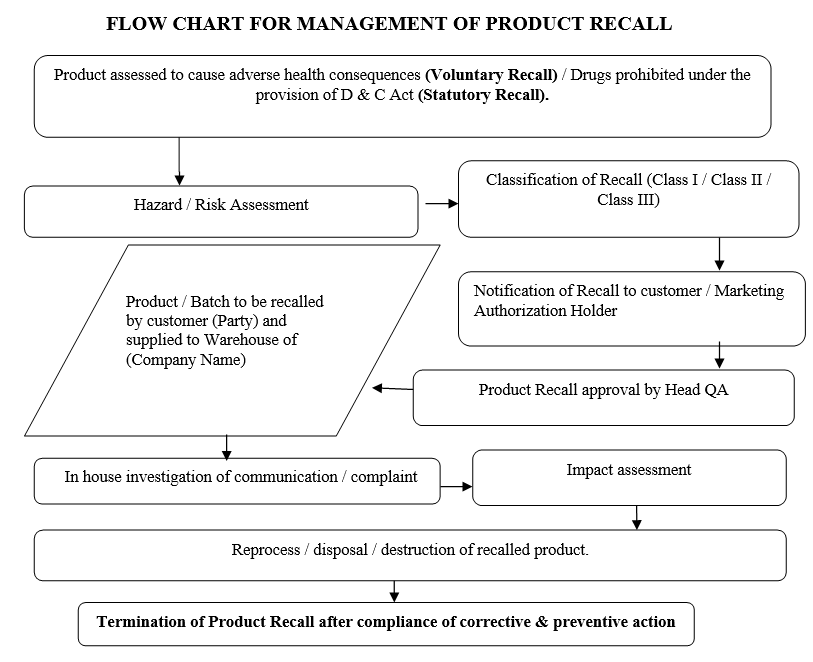
FLOW CHART FOR MANAGEMENT OF PRODUCT RECALL
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/product-recall/
- REFERENCE:
CDSCO Guidelines on recall and rapid alert system for drugs.
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
| Annexure-I | Product Recall Notice |
| Annexure-II | Logbook for Product Recall |
| Annexure-III | Recall Material Destruction Note |
| Annexure-IV | Product Recall Summary Report |
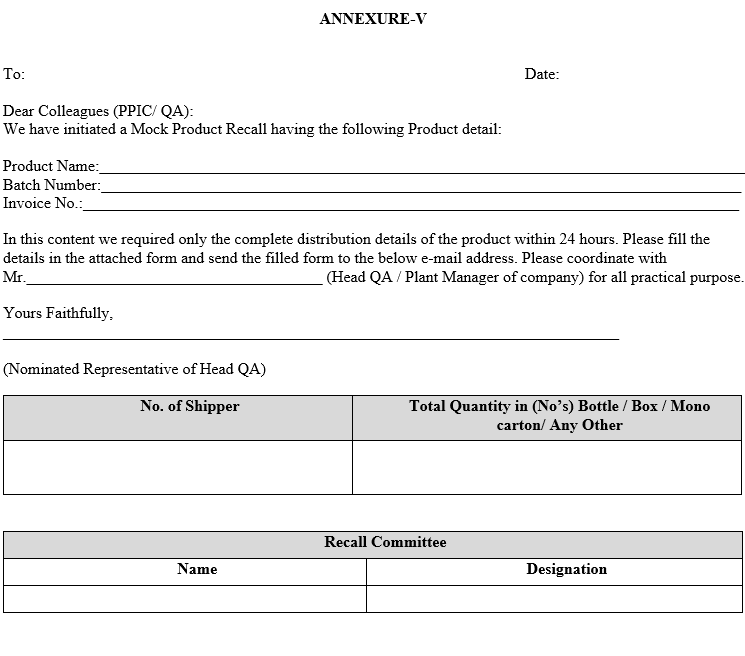
| Annexure-V | Mock Recall Letter |
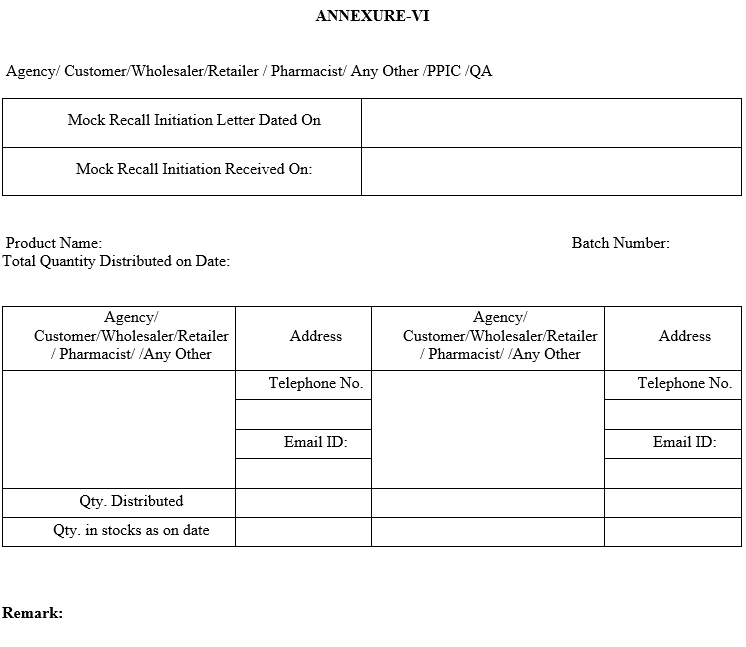
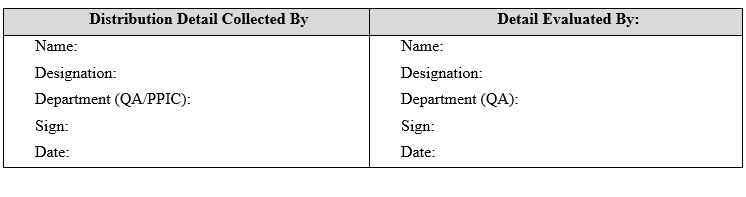
| Annexure-VI | Distribution Details |
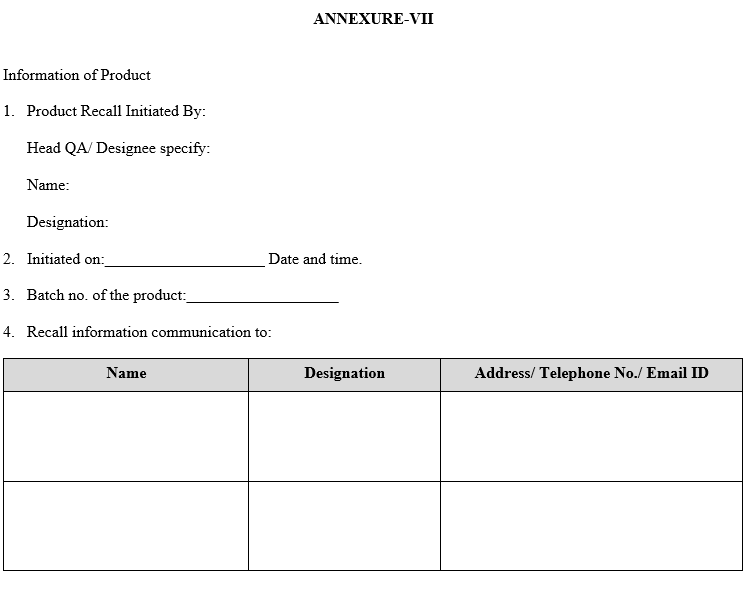
| Annexure-VII | Mock Product Recall Report |
- DISTRIBUTION:
· Controlled Copy No. 01 : Head Quality Assurance
· Controlled Copy No. 02 : Head Quality Control
· Controlled Copy No. 03 : Head Production
· Controlled Copy No. 04 : Head Warehouse
· Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| SOP | : | Standard Operating Procedure |
| No. | : | Number |
| QA | : | Quality Assurance |
| Sr. | : | Senior |
| PRC | : | Product Recall |
| CDSCO | : | Central Drugs Standard Control Organisation |
- REVISION HISTORY:
- CHANGE HISTORY LOG
Revision No. Details of Changes Reason for Change Effective Date 00 New SOP Not Applicable To be written manual
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/product-recall/
ANNEXURE-I
ANNEXURE-II
| S. No. | PCR No. | Date Receipt | Product Name | Batch No. | Mfg. Date | Exp. Date | Reason for Recall | Class (I/II/III) | Quantity Recalled | Closure of Recall (Sign & Date) | Date of Destruction of Recalled Material | Sign & Date | Remarks |
ANNEXURE-III
ANNEXURE-IV
ANNEXURE-V
ANNEXURE-VI
ANNEXURE-VII

Click the link for download word file copy of this document: https://pharmaguidehub.com/product/product-recall/
1. What is a product recall in pharma?
A product recall in pharma occurs when a company voluntarily removes a drug or medical device from the market because it poses a potential safety risk to patients. This can be due to various reasons like manufacturing defects, unexpected side effects, or contamination.
2. What are the different types of product recalls in pharma?
- Class I recalls: These involve the highest risk, with potential for serious health problems or death.
- Class II recalls: These involve a moderate risk, with potential for temporary or reversible adverse effects.
- Class III recalls: These involve a low risk, with unlikely adverse effects but potential for product failure or minor problems.
3. What are the common reasons for drug recalls?
- Manufacturing errors: Contamination, incorrect dosage, incorrect labeling.
- Unexpected side effects: Not identified during clinical trials or identified after release.
- Stability issues: Degradation of the drug over time, loss of potency.
- Counterfeiting: Fake drugs posing safety and efficacy concerns.
4. How does a company initiate a product recall?
- Identify the potential safety risk.
- Notify the regulatory authorities (FDA, EMA, etc.).
- Issue a public recall notice, informing healthcare providers and patients about the risk and instructions for returning the product.
- Implement a recall plan to collect and dispose of the recalled product safely.
5. What are the consequences of a product recall for a pharmaceutical company?
- Financial losses: Costs of recall, potential lawsuits, damaged reputation.
- Regulatory action: Fines, warnings, or even product withdrawal from the market.
- Loss of patient trust: Damage to brand image and public perception.
6. How can patients stay informed about drug recalls?
- Subscribe to FDA and EMA recall notification lists.
- Check the company’s website and social media for recall announcements.
- Contact their healthcare provider or pharmacist for information about specific drugs.
7. What should patients do if they have the recalled product?
- Stop using the product immediately.
- Contact their healthcare provider for instructions.
- Return the product according to the recall notice instructions.
8. Can patients sue a pharmaceutical company for a product recall?
Yes, patients may be eligible for lawsuits if they experience harm due to the recalled product. This depends on various factors like individual circumstances and the specific case.
9. How can pharmaceutical companies prevent product recalls?
- Implement robust quality control measures throughout manufacturing and distribution.
- Conduct thorough clinical trials to identify potential safety risks.
- Monitor post-market safety data and promptly address any concerns.
- Develop and implement effective risk management plans.
10. Are product recalls increasing in the pharma industry?
Data shows a slight upward trend in recalls in recent years. This could be due to increased scrutiny by regulators, improved detection methods, or more complex drug manufacturing processes.
11. How transparent are pharmaceutical companies about product recalls?
Transparency varies depending on the company and the severity of the recall. Some companies provide detailed information quickly, while others may be slower to disclose details.
12. What role do regulatory agencies play in product recalls?
Regulatory agencies like the FDA and EMA oversee product safety and enforce recall regulations. They investigate potential risks, monitor recalls, and take action against companies that fail to comply.
13. Are there international guidelines for product recalls?
Yes, the ICH (International Council for Harmonisation) provides guidelines for harmonizing global approaches to product recalls. These guidelines aim to ensure consistency and effectiveness of recalls across different countries.
14. How can technology be used to improve product recall management?
Technologies like blockchain and track-and-trace systems can enhance product traceability and facilitate faster, more efficient recalls.
15. What is the future of product recalls in pharma?
The industry is continuously working to improve recall processes and prevent safety issues. Continued research, technological advancements, and international collaboration are crucial for ensuring patient safety and building trust in the pharmaceutical industry.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/product-recall/