- OBJECTIVE:
- To lay down a procedure for training and to establish a mechanism for evaluation of an Analyst on General Analytical Techniques.
- SCOPE:
This SOP is applicable to procedure for training and to establish a mechanism for evaluation of an Analyst on General Analytical Techniques.
- RESPONSIBILITY:
- Officer/Executive/Designee Quality Control – Shall be responsible for operation as per SOP.
- Head/Designee Quality Control – Shall be responsible for ensuring compliance as per SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- The analysts having previous experience should fill the QC Competency Checklist for Recruitees while entering the QC lab at the time of Induction and checklist not applicable for the analysts whose not having any previous experience.
- All the analysts undertaking analyst qualification shall undergo training and qualify on relevant Standard Operating Procedures (SOPs) and General analytical techniques.
- The Person undergoing Analyst Qualification should have below list `Acceptance Criteria for Qualification of Analyst` for General procedure and for Acceptance Criteria.
- The training on analytical techniques shall be carried out under the supervision of the Senior Executive or an analyst already trained in that technique, by following the STP / SOP and using already approved product / material as test sample.
- The sample for analyst qualification shall be collected from control sample as per respective SOP.
- To qualify on the analytical technique the analyst shall perform the test.
- The results shall be comparable with the results already reported and meet the acceptance criteria.
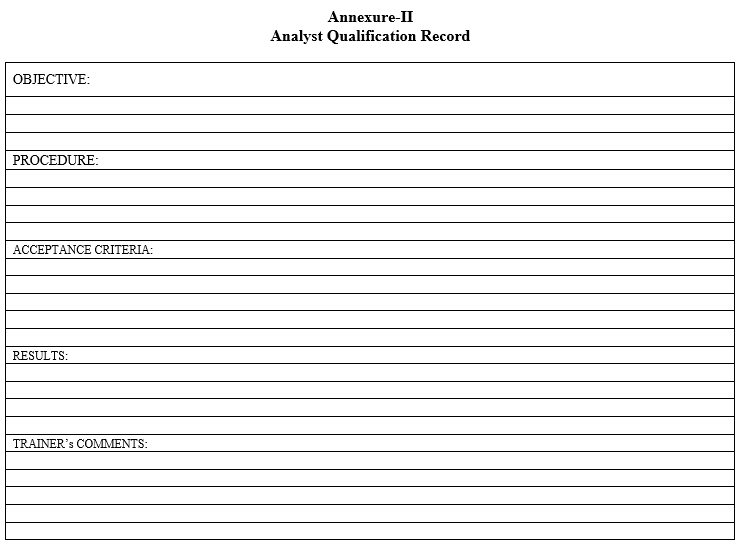
- The training on general analytical technique and evaluation shall be documented in the analyst qualification record as per the Format `Analyst Qualification Record’ under the following headings:
- OBJECTIVE
- PROCEDURE
- ACCEPTANCE CRITERIA
- RESULTS
- TRAINER’S COMMENTS
- The trainer shall review and evaluate the data generated by the analyst.
- If the above mentioned details are found satisfactory, it can be concluded that the analyst is qualified.
- In case of any discrepancy or abnormality observed, it can be investigated, counseled by omitting the mistakes, training shall be repeated and again evaluated.
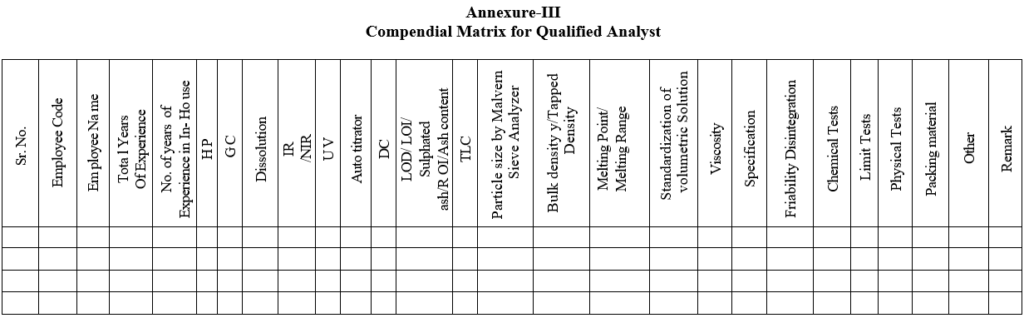
- If the Performance found satisfactory enter the same in Compendial Matrix for Qualified Analyst’.
- Apart from the technical training related to Quality Control operation, all additional training is outlined in the SOP by the training department.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/qualification-of-analyst-in-quality-control/
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | QC Competency Checklist for Recruitees |
| Annexure-II | Analyst Qualification Record |
| Annexure-III | Compendial Matrix for Qualified Analyst |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| NMT | : | Not More Than |
| STP | : | Standard Testing Procedure |
| LOD | : | Limit of Detection |
| LOQ | : | Limit of Quantification |
| CP | : | Common Procedure |
| GTP | : | General Test Procedure |
| HPLC | : | High Performance Liquid Chromatography |
| QC | : | Quality Control |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
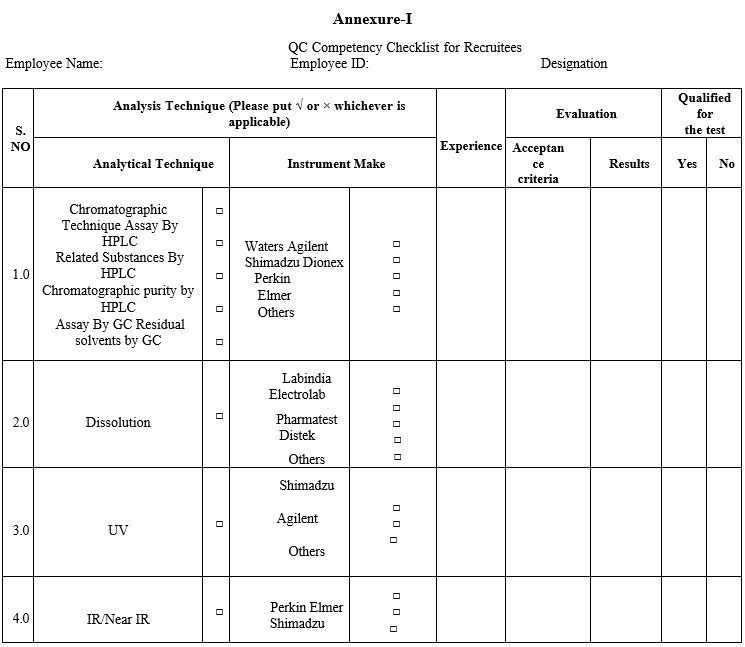
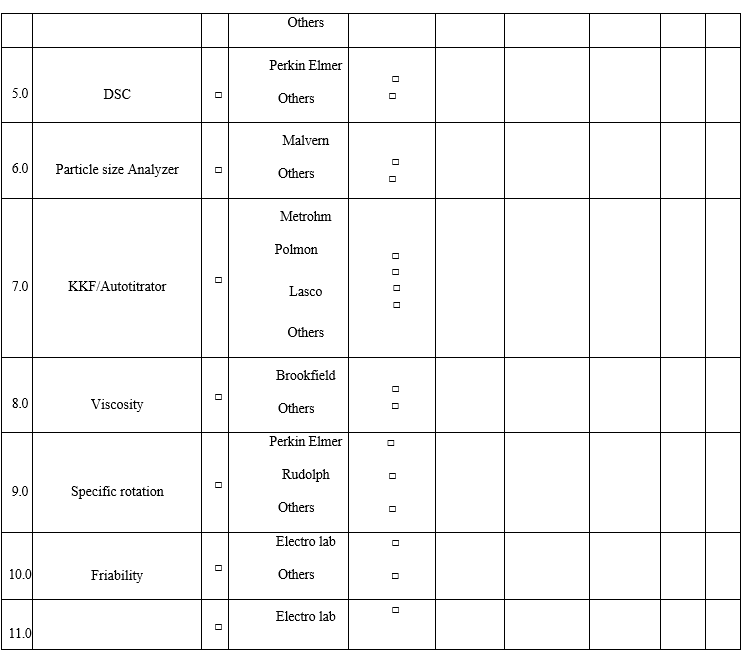
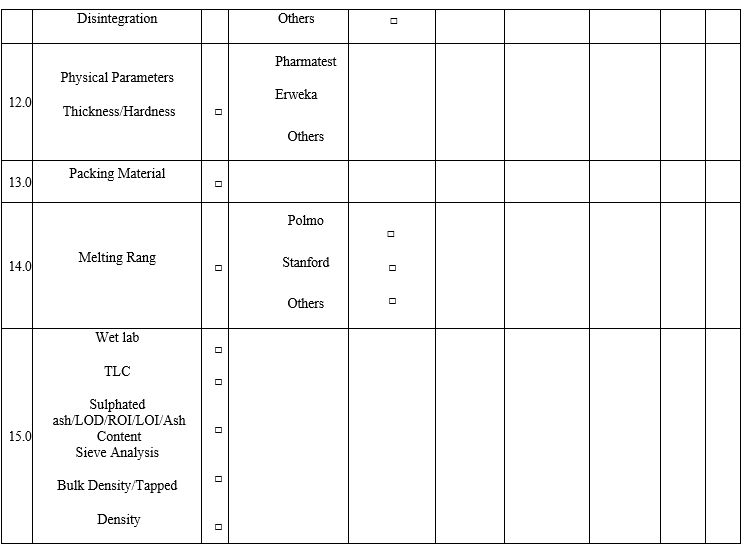
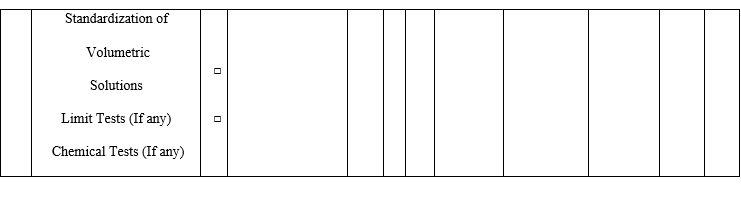
Annexure-II
Analyst Qualification Record
Annexure-III
Compendial Matrix for Qualified Analyst
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/qualification-of-analyst-in-quality-control/
