- PROCEDURE FOR MICROBIOLOGIST QUALIFICATION:
- All the new entrant personnel to Microbiology laboratory shall be trained properly with respect to analytical techniques and challenging studies.
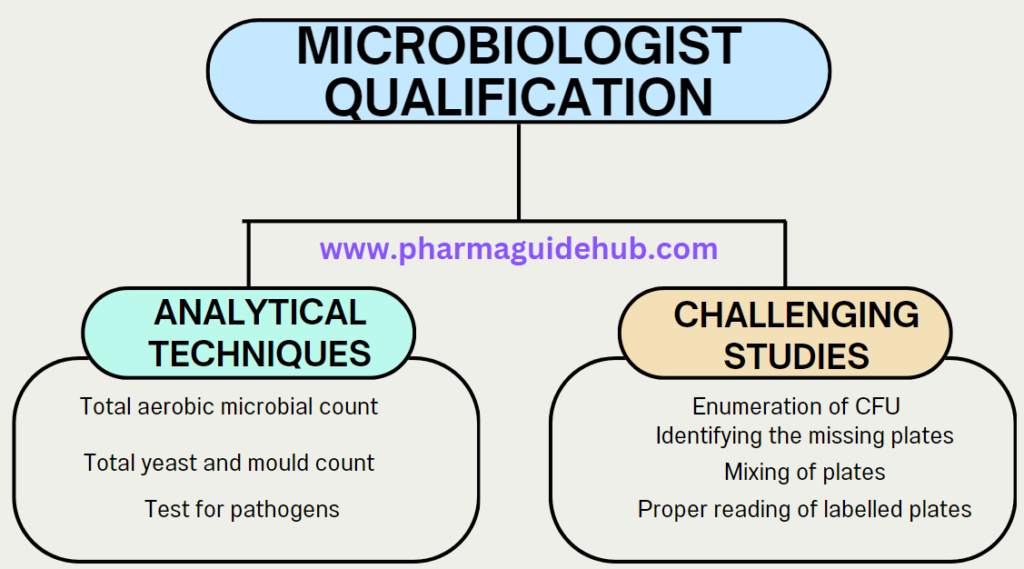
- Analytical techniques include:
- Total aerobic microbial count,
- Total yeast and mould count,
- Test for pathogens.
- Challenging studies include:
- Enumeration of colony forming units (CFU’s),
- Identifying the missing plates,
- Mixing of plates,
- Proper reading of labelled plates.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/qualification-of-analyst-microbiologist-in-quality-control/
- EVALUATION OF ANALYTICAL TECHNIQUES:
- To evaluate the analytical techniques of the analyst, he/she shall be provided with unknown samples.
- The analyst who is to be qualified will be provided with the department copy of the MLT test procedure.
- After clarifying the doubts of the analyst if any, he/she is provided with the microbial limit test questionnaire (Format – I)
- After the analyst is qualified in the written test, he/she is provided with the sample required to perform the microbial limit testing.
- The trainer will prepare and provide the analyst 3 sets of unknown samples in 250 ml and 100 ml bottles, each set containing two samples in that one for total aerobic microbial count purpose and another for specified microorganisms purpose.
- Each 250 ml bottle will filled with 100 ml of saline samples and 10 ml of saline sample in 100 ml bottle.
- All three set bottle will sterilized by autoclaving.
- After sterilization, label the saline sample bottles as Lot 01, Lot 02 and Lot 03.
- Aseptically open one set of bottles from any one of the above lots and inoculate 10-100 cells of culture suspension of any one of the microorganism for total aerobic microbial count and one of the microorganism for specified pathogen.
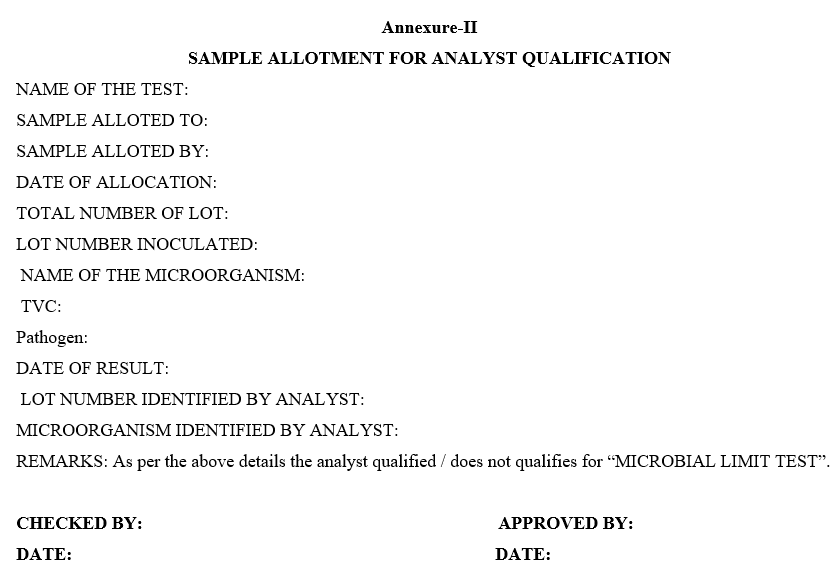
- Record the lot number and name of the microorganism as per Format – II (Sample allotment for analyst qualification) and allot the samples for analyst to be qualified.
- The microbiologist who is to be qualified is then given the three samples to perform MLT test.
- The analyst shall enter in to the testing area as per SOP.
- The analyst shall perform the total aerobic microbial count by filtration method and pathogen test as per GTP.
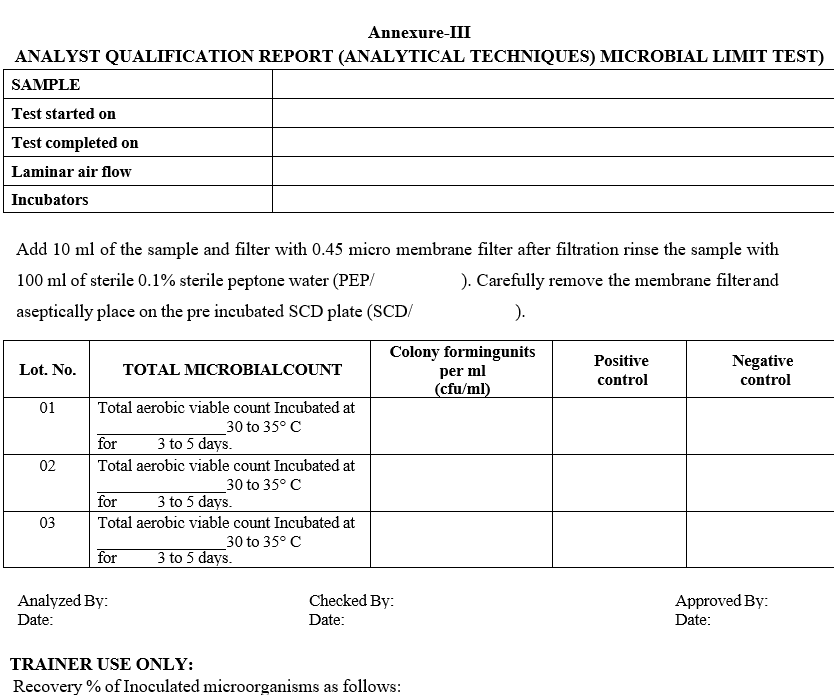
- The analyst should record the results in Format – III {Analyst qualification report (Analytical techniques)}
- EVALUATION THROUGH CHALLENGING STUDIES: This challenging study plan is conducted to evaluate the Microbiologists in different methods for getting the data integrity of the Microbiological analysis performed in the Microbiology Laboratory.
- Challenging the Microbiologists for enumerating the CFU’s:
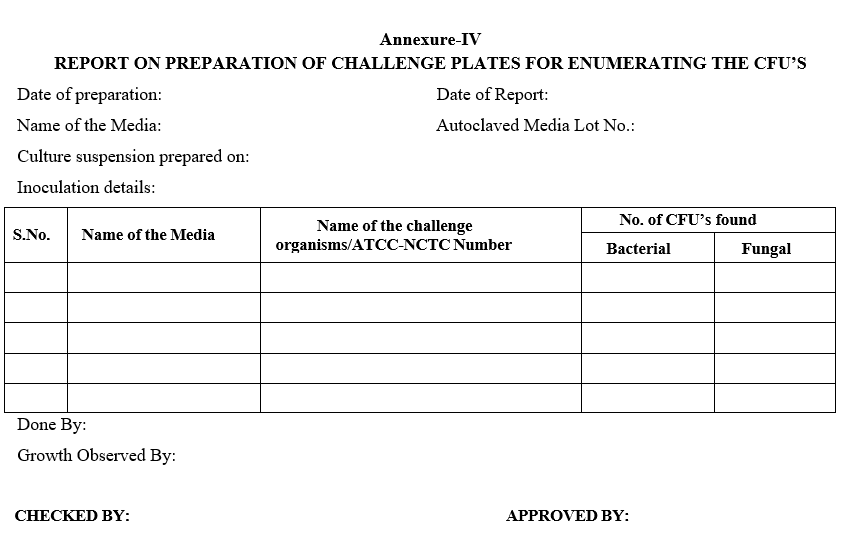
- Head – Microbiology shall prepare the bacterial and fungal colony plates by inoculating culture suspension having <100 CFU/0.1 ml on to the surface of the SCD and SDA agar plates respectively.
- Incubate the SCD plates at 30 – 35°C for 24 hrs, incubate SDA plates at 20 – 25°C for 72 hrs which shall be used in challenge study. The inoculation details shall be recorded as per Format – IV.
- Select the plate having the required number of CFU crossing the alert level/ action level as applicable.
- Thus selected plates shall be replaced in the incubator, by preserving original exposed plate in a separate incubator by the Head – Microbiology.
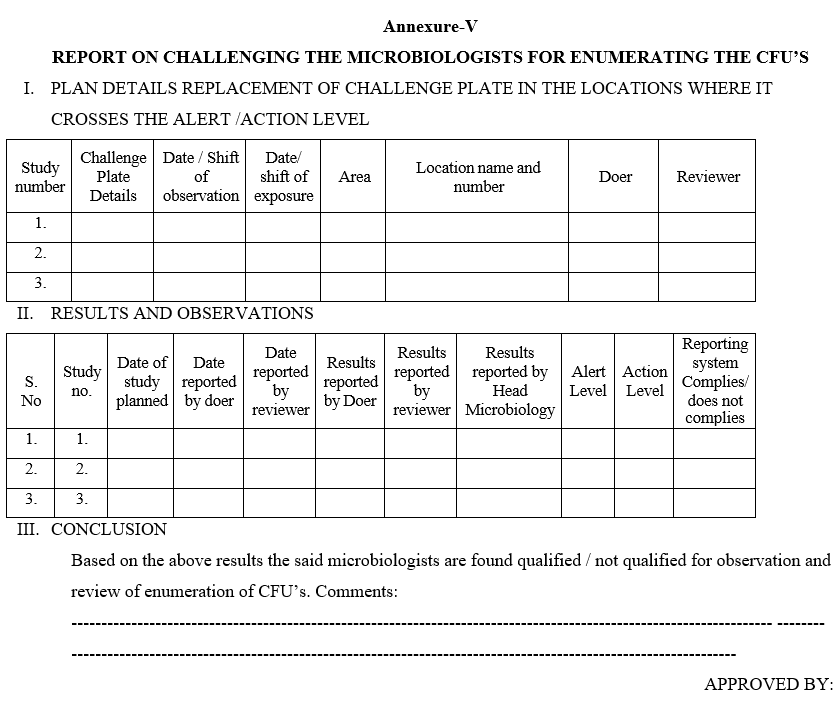
- Head Microbiology shall note down both the details of the plate taken and plate replaced. Viz; Name of the Location, area, date of exposure etc. as per the Format – V.
- After the specified incubation period the Microbiologist (doer) as per the routine observations, he has to check the plates and record the observations.
- Reviewer has to check the same plates and observations shall be noted.
- The reviewer has to come and report to the Head – Microbiology.
- Head Microbiology shall ensure the same plate observations reported by the doer and reviewer.
- Head Microbiology shall interview the doer and reviewer for the observed counts.
- Head Microbiology then check the original plate, which is incubated in other incubator and note down the results in the respective Format by correcting the data entered by the doer and reviewer put appropriate comments as “The counts are observed as a part of challenge study of observation and review mechanism and the actual results are now reported by Head – Microbiology”.
- The above exercise shall be performed in three different days (not necessarily to cover all the microbiologists) and shall be planned for other microbiologist as and when required.
- Challenging the Microbiologists for identifying the missing of plates:
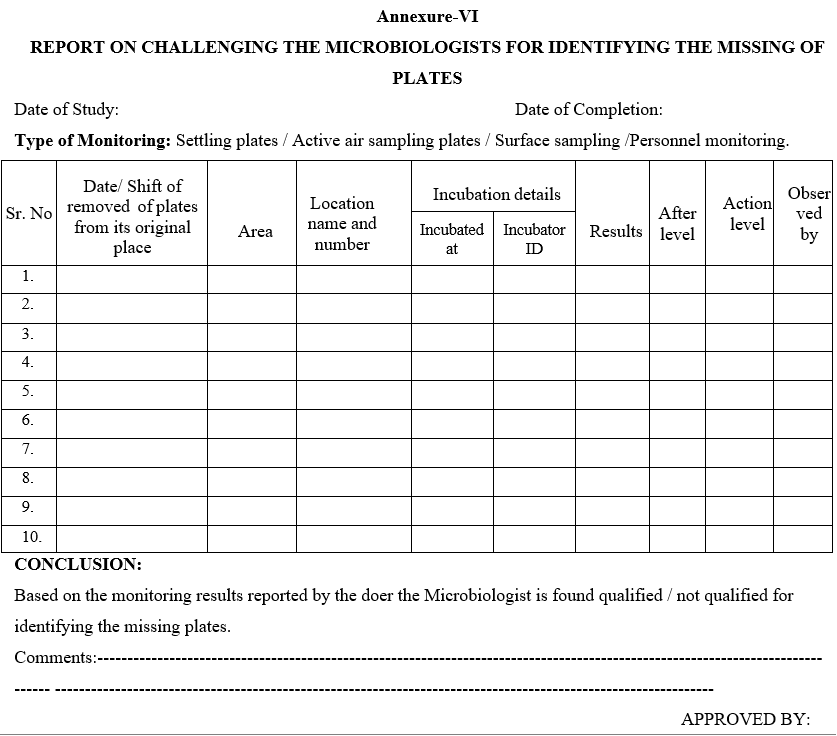
- Head – Microbiology shall remove one or two plates from the monitored plates and incubate those plates in another incubator by noting down the details of the plate taken Viz; Name of the Location, area, date of exposure etc. as per the Format – VI.
- After the specified incubation period, the Microbiologist (doer) as per the routine observations, he has to check the plates and record the observations.
- Reviewer has to check the same plates and observations shall be noted.
- The reviewer has to come and report to the Head – Microbiology as some of the plates, they could not find out in the incubator and should declare as those plates were missing.
- Head Microbiology shall ensure the same plate observations reported by the doer and reviewer.
- Head Microbiology shall interview the doer and reviewer for the missing plates.
- Head Microbiology then check the original plate, which is incubated in other incubator and note down the results in the respective Format by correcting the data entered by the doer and reviewer put appropriate comments as “The plates are removed and as a part of challenge study of observation and review mechanism and the actual results are now reported by Head – Microbiology”.
- Challenging the Microbiologists for identifying the extra plates (mixing of plates):
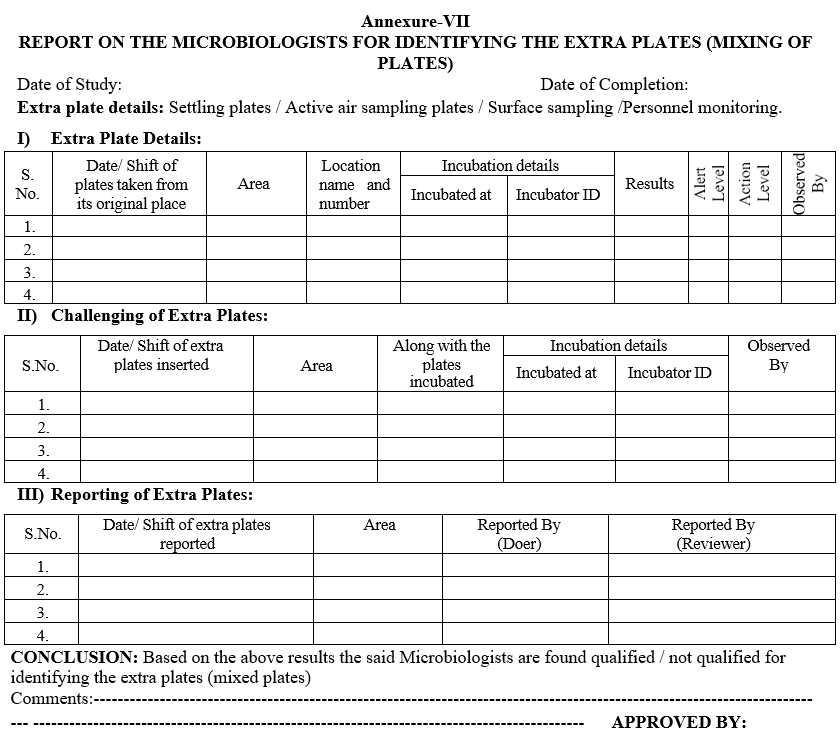
- Head – Microbiology shall insert one or two plates of other area and incubate those plates in the same incubator by noting down the details of the plate taken Viz: Name of the Location, area, date of exposure etc. as per the Format – VII.
- After the specified incubation period, the Microbiologist (doer) as per the routine observations, he has to check the plates and record the observations.
- Reviewer has to check the same plates and observations shall be noted.
- The reviewer has to come and report to the Head – Microbiology as some other area plates were mixed and should show to Head-Microbiology.
- Head Microbiology shall ensure the same plate observations reported by the doer and reviewer.
- Head Microbiology shall interview the doer and reviewer for the mixing plates.
- Head Microbiology then replaces those plates at the original place, where he took for the challenge study.
- Note down the results in the respective Format by correcting the data entered by the doer and reviewer put appropriate comments as “The plates are inserted as a part of challenge study of observation and review mechanism and then replaced to its original place by Head – Microbiology”.
- Challenging the Microbiologists for proper reading of labeled plates and recording the observations:
- After the specified incubation period of the monitored plates the Microbiologist (doer) as per the routine observations, he has to check the plates and record the observations.
- Reviewer has to check the same plates and observations shall be noted.
- Head – Microbiology shall re-verify the observed plates in the decontamination room and cross verify the results reported by the Microbiologist & reviewer as per the Format – VIII.
- Head Microbiology shall evaluate all the plates observed by the doer and reviewer is accurate and confirming to the integrity of the data reported.
- Head-Microbiology shall submit his observations and doer, reviewer observations to Head-Quality Assurance.
- Head-Quality Assurance shall evaluate the data reported by the Head-Microbiology, doer and reviewer and assures on the data integrity of the test results.
- Frequency:
- If the analyst is in long leave (above two months) he/she has to be re-qualified.
- For all the new entrant to microbiology analyst qualification shall be performed.
- The above exercise shall be performed in three different days (not necessarily to cover all the microbiologist) and shall be planned for different microbiologist as and when required.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/qualification-of-analyst-microbiologist-in-quality-control/
- Acceptance Criteria:
| Test | Acceptance criteria |
| Microbial limit test | The negative control plates should not show any sort of growth of microorganisms.Inoculated microorganism should be identified rightly. Recovery of the inoculated microorganisms should be more than 70%.The inoculated Pathogen should be identified. |
| Test | Acceptance criteria |
| Challenging the Microbiologists for enumerating the CFU’s | The doer and the reviewer should inform to the department head regarding the area which shows more counts.Head in turn should verify the name of the area and counts of the exposed plate. |
| Challenging the Microbiologists for identifying the missing of plates | The doer and the reviewer should inform to the department head regarding the missing of the plate. Head in turn should verify Name of the Location, area, date of exposure of the exposed plate. |
| Challenging the Microbiologists for identifying the extra plates (mixing of plates) | The doer and the reviewer should inform to the department head regarding the mixing of the plate.Head in turn should verify Name of the Location, area, date of exposure of the exposed plate. |
| Challenging the Microbiologists for proper reading of labeled plates and recording the observations | The doer and the reviewer should report the results to the department head. Head in turn should verify all the plates and the report to evaluate the integrity of reporting. |
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Microbial limit test questionnaire |
| Annexure-II | Sample allotment for analyst qualification |
| Annexure-III | Analyst qualification report (Analytical techniques) |
| Annexure-IV | Report on Preparation of Challenge plates for enumerating the CFU’s |
| Annexure-V | Report on Challenging the Microbiologists for enumerating the CFU’s |
| Annexure-VI | Report on Challenging the Microbiologists for identifying the missing of plates |
| Annexure-VII | Report on the Microbiologists for identifying the extra plates (mixing of plates) |
| Annexure-VIII | Report on Challenging the Microbiologists for proper reading of labelled plates |
| Annexure-IX | Certificate of microbiologist |
- ABBREVIATIONS:
| No. | : | Number |
| SCD | : | Soyabean casein digest agar |
| cfu | : | colony-forming unit |
| SDA | : | Sbouraud’s dextrose agar |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/qualification-of-analyst-microbiologist-in-quality-control/
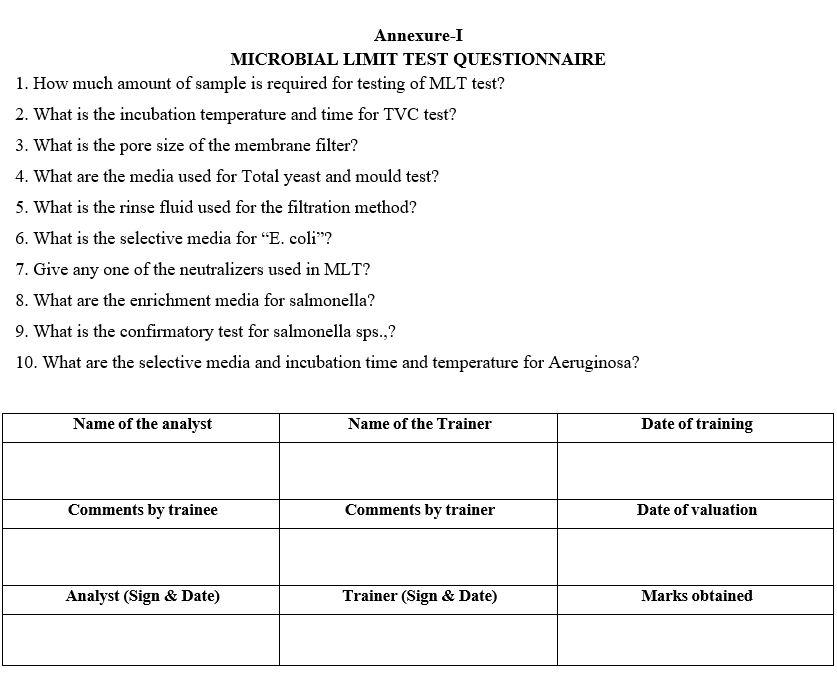
Annexure-I
MICROBIAL LIMIT TEST QUESTIONNAIRE
Annexure-II
SAMPLE ALLOTMENT FOR ANALYST QUALIFICATION
Annexure-III
ANALYST QUALIFICATION REPORT (ANALYTICAL TECHNIQUES) MICROBIAL LIMIT TEST
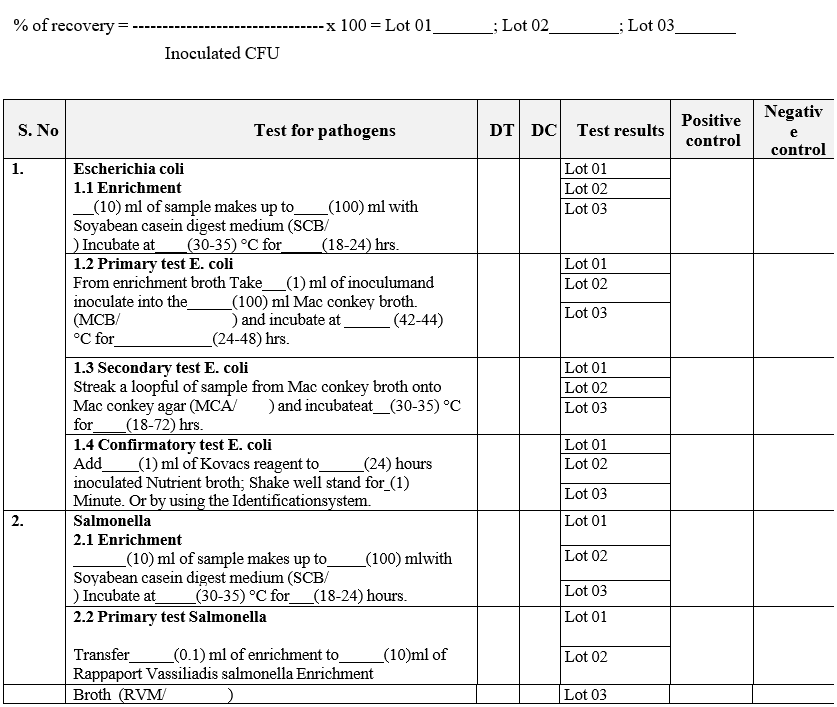
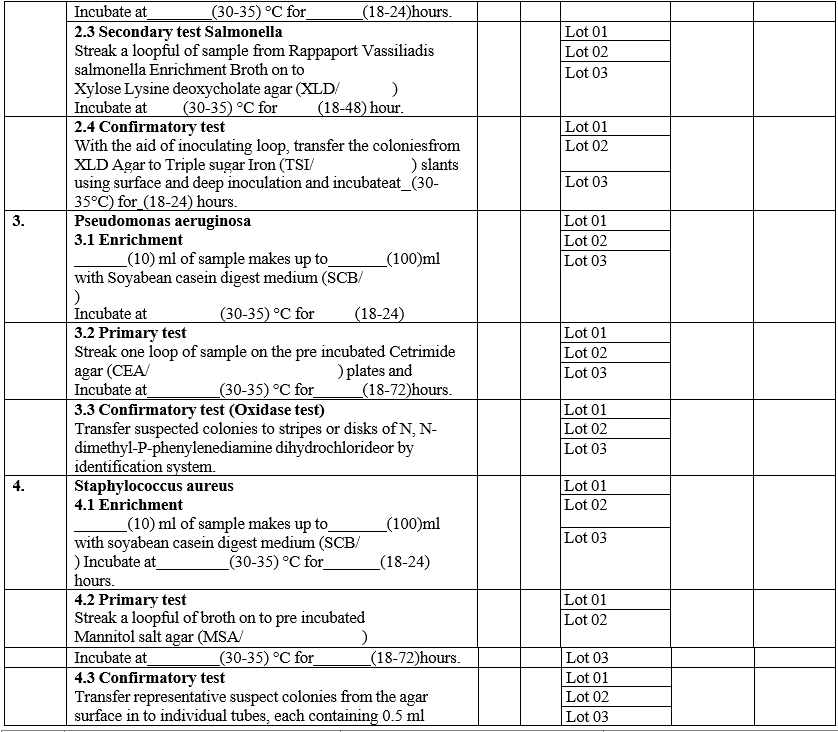
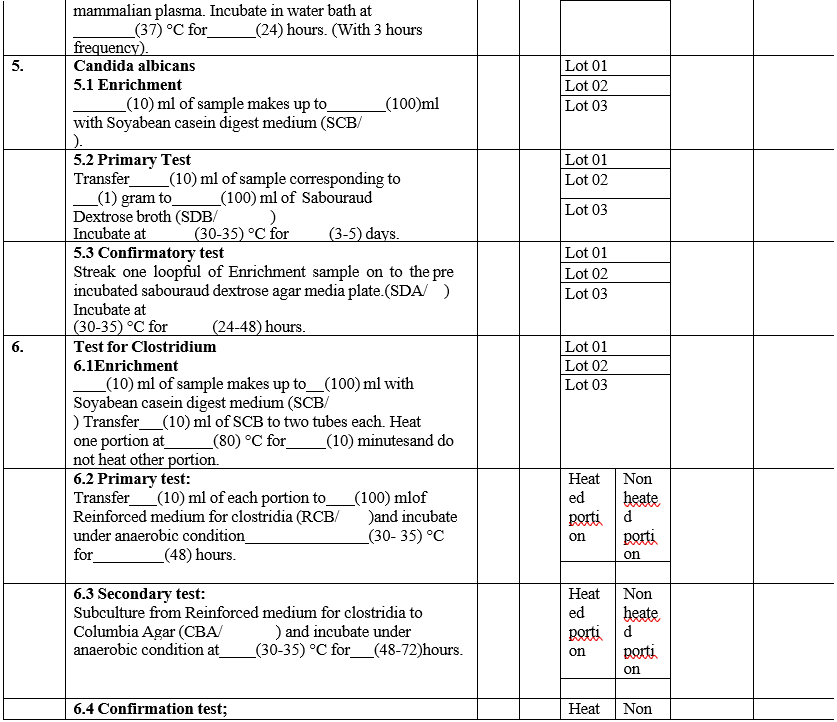
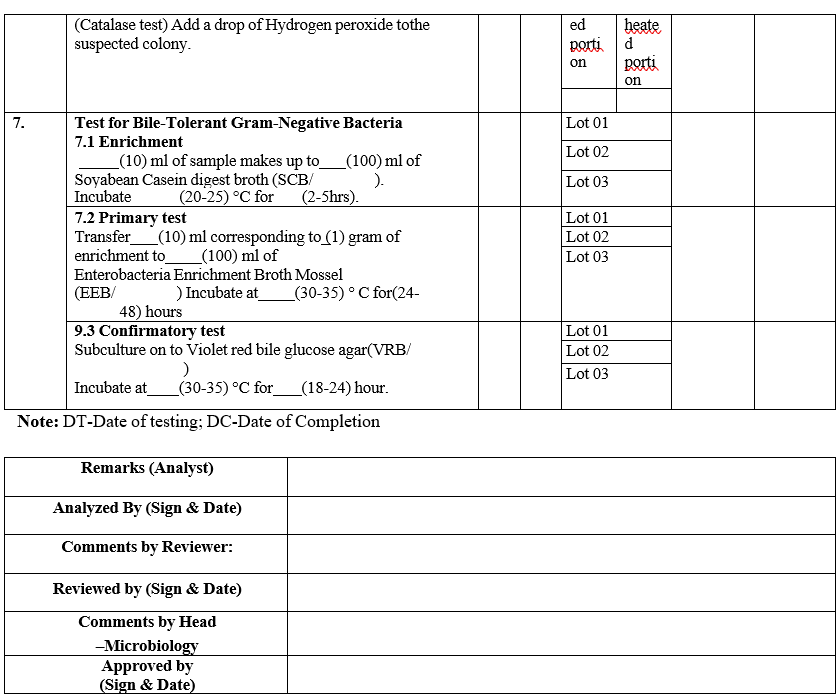
Annexure-IV
REPORT ON PREPARATION OF CHALLENGE PLATES FOR ENUMERATING THE CFU’S
Annexure-V
REPORT ON CHALLENGING THE MICROBIOLOGISTS FOR ENUMERATING THE CFU’S
Annexure-VI
REPORT ON CHALLENGING THE MICROBIOLOGISTS FOR IDENTIFYING THE MISSING OF PLATES
Annexure-VII
REPORT ON THE MICROBIOLOGISTS FOR IDENTIFYING THE EXTRA PLATES (MIXING OF PLATES)
Annexure-VIII
Report on Challenging the Microbiologists for proper reading of labelled plates
Annexure-IX
CERTIFICATE OF MICROBIOLOGIST

Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/qualification-of-analyst-microbiologist-in-quality-control/

