In the pharmaceutical industry, a quality manual is a critical document that describes the company’s entire Quality Management System (QMS). It serves as a roadmap for ensuring consistent production of safe and effective medications.
A pharmaceutical quality manual is a foundational document outlining an organization’s commitment to quality and compliance. It details the quality management system (QMS), encompassing policies, procedures, and responsibilities. This manual ensures adherence to regulatory requirements like GMP, outlining how products are consistently manufactured, tested, and released. It covers aspects like document control, change management, and deviations, fostering a culture of quality. Risk management and continuous improvement are also emphasized, ensuring product safety and efficacy. The manual serves as a guide for employees, auditors, and regulatory bodies, promoting transparency and accountability across all operations.
QUALITY MANUAL (PART 1 OF 2)

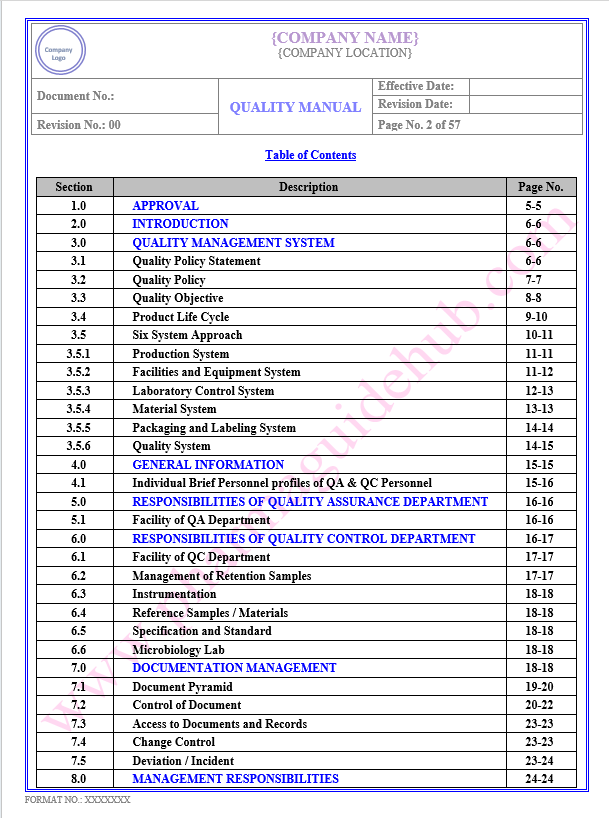
Key Parameter of Below Page:
Table of contents
Find the below page for content
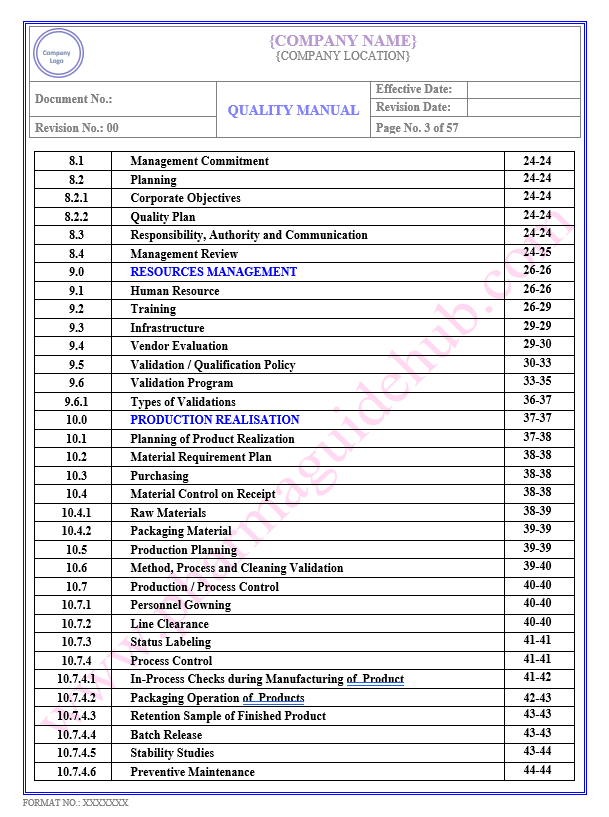
Key Parameter of Below Page:
Table of contents
Find the below page for content
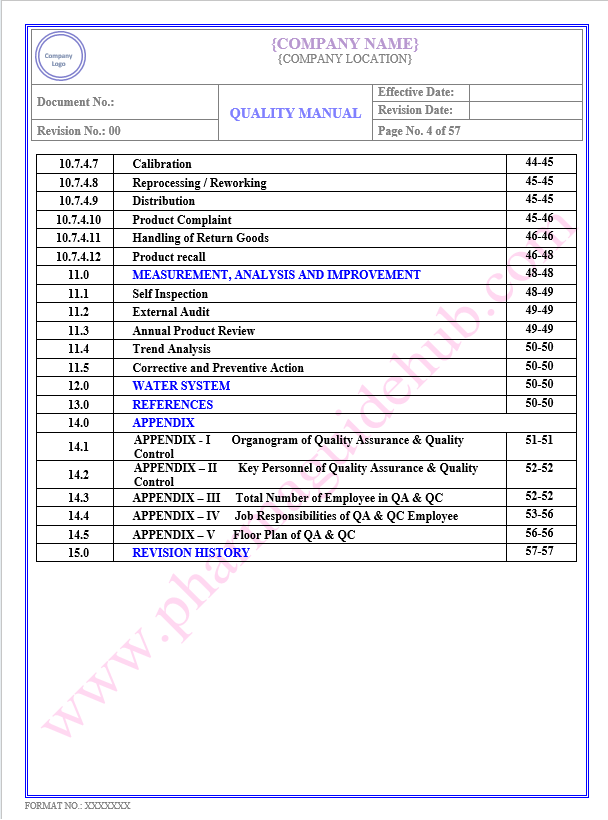
Key Parameter of Below Page:
Table of contents
Find the below page for content
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quality-manual/
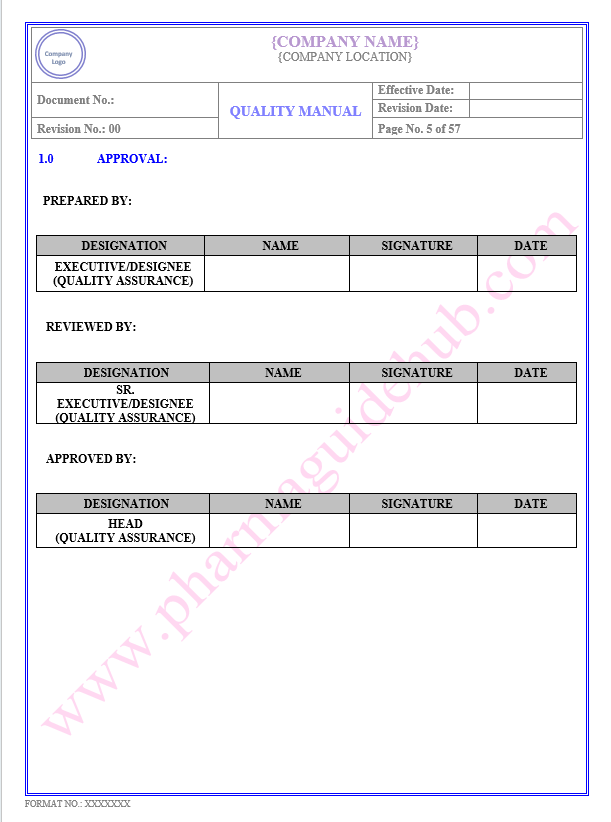
Key Parameter of Below Page:
Approval
Find the below page for content
Key Parameter of Below Page:
Introduction
Quality Management System
Find the below page for content
Key Parameter of Below Page:
Quality Policy
Find the below page for content

Key Parameter of Below Page:
Quality Objective
Find the below page for content
Key Parameter of Below Page:
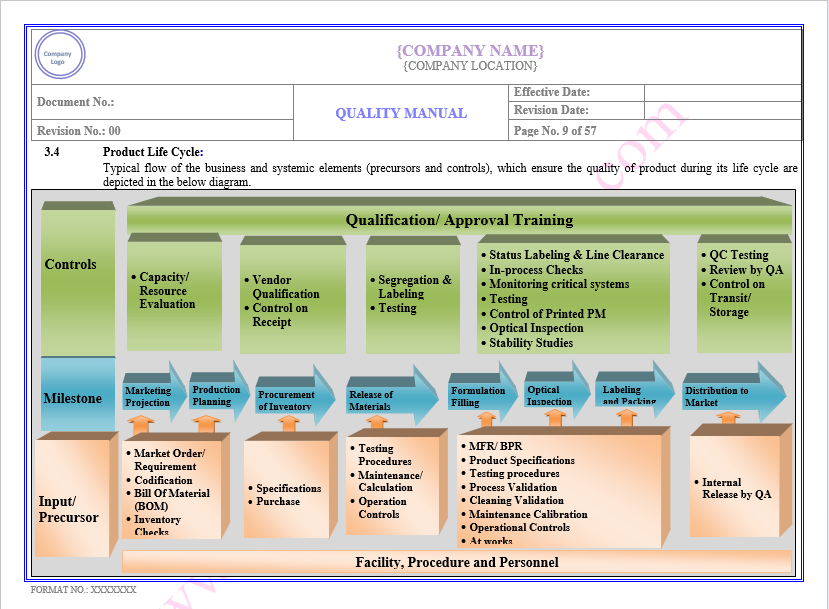
Product Life Cycle
Find the below page for content
Key Parameter of Below Page:
Six System Approach
Find the below page for content

Key Parameter of Below Page:
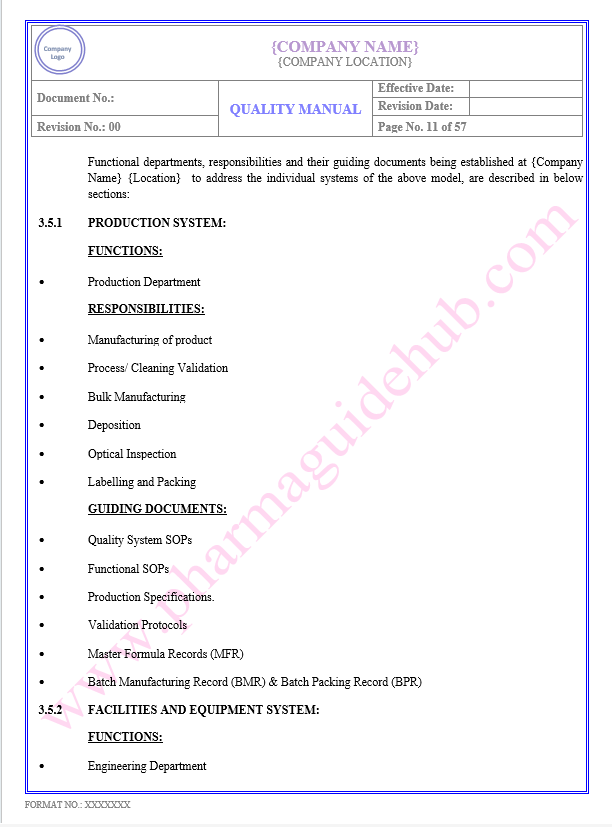
Production System
Facility and Equipment System
Find the below page for content
Key Parameter of Below Page:
Laboratories Control System
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quality-manual/

Key Parameter of Below Page:
Material System
Find the below page for content

Key Parameter of Below Page:
Packing and Labeling System
Quality System
Find the below page for content

Key Parameter of Below Page:
General Information
Find the below page for content
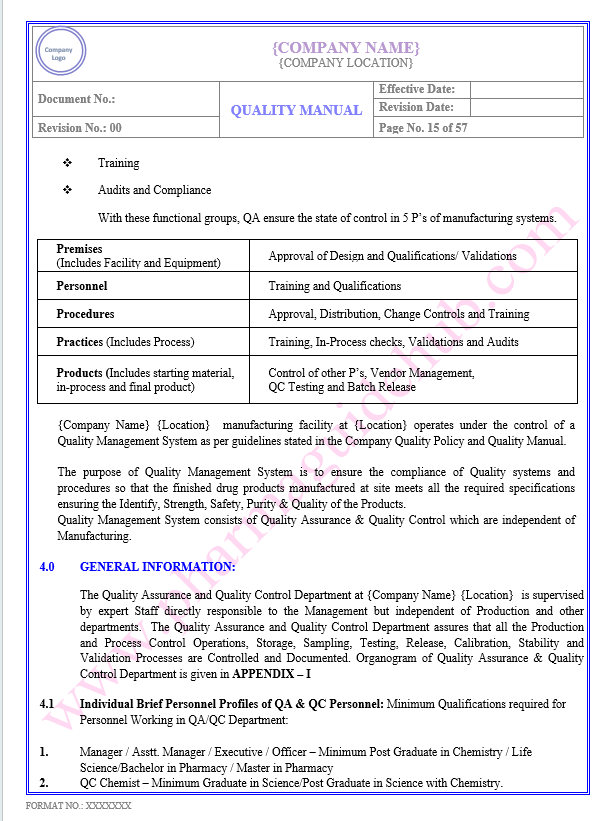
Key Parameter of Below Page:
Responsibility of Quality Assurance Department
Responsibility of Quality Control Department
Find the below page for content

Key Parameter of Below Page:
Responsibility of Quality Control Department
Find the below page for content
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quality-manual/

Key Parameter of Below Page:
Responsibility of Quality Control Department
Document Management
Find the below page for content
Key Parameter of Below Page:
Document Management
Find the below page for content
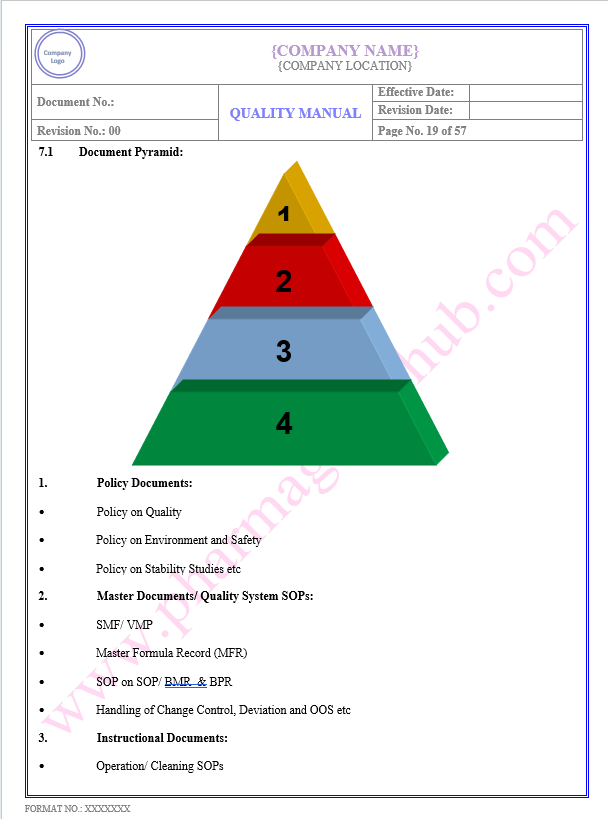
Key Parameter of Below Page:
Document Management
Find the below page for content
Key Parameter of Below Page:
Document Management
Find the below page for content
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quality-manual/

Key Parameter of Below Page:
Document Management
Find the below page for content

Key Parameter of Below Page:
Document Management
Find the below page for content

Key Parameter of Below Page:
Document Management
Management Responsibility
Find the below page for content
Key Parameter of Below Page:
Document Management
Find the below page for content
Key Parameter of Below Page:
Document Management
Resource Management
Find the below page for content

Key Parameter of Below Page:
Resource Management
Find the below page for content
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quality-manual/
Click the link for part 2 of 2
https://pharmaguidehub.com/quality-manual-part-2-of-2/






The text highlights the importance of a quality manual in the pharmaceutical industry, which is indeed crucial for maintaining high standards. However, I wonder how often these manuals are updated to reflect new regulations or advancements in technology. The emphasis on risk management and continuous improvement is commendable, but does this translate into real-world practices across all organizations? It’s interesting to see how the manual serves as a guide for employees, auditors, and regulatory bodies, but how effective is it in fostering a genuine culture of quality? The focus on document control and change management is essential, but are there common challenges in implementing these systems? Lastly, how do companies ensure that all employees, especially new hires, fully understand and adhere to the guidelines outlined in the manual?
We have integrated libersave into our regional voucher system. It’s fantastic how easily it consolidates various providers on a single platform.
The quality manual is indeed a cornerstone in the pharmaceutical industry, ensuring that every step of the production process adheres to the highest standards. It’s impressive how it covers everything from document control to risk management, fostering a culture of continuous improvement. However, I wonder how often these manuals are updated to keep up with evolving regulations and technological advancements. Do companies face challenges in maintaining such comprehensive documentation, especially in a fast-paced industry? It’s also interesting how the manual serves as a guide not just for employees but also for auditors and regulatory bodies. How do companies ensure that every employee, regardless of their role, fully understands and implements the guidelines outlined in the manual? Lastly, how effective is the manual in real-world scenarios when unexpected deviations occur?
We have integrated libersave into our regional voucher system. It’s amazing how easily it allows us to consolidate various providers on a single platform.