QUALITY MANUAL (PART 2 OF 2)
In the pharmaceutical industry, a quality manual is a critical document that describes the company’s entire Quality Management System (QMS). It serves as a roadmap for ensuring consistent production of safe and effective medications.
A pharmaceutical quality manual is a foundational document outlining an organization’s commitment to quality and compliance. It details the quality management system (QMS), encompassing policies, procedures, and responsibilities. This manual ensures adherence to regulatory requirements like GMP, outlining how products are consistently manufactured, tested, and released. It covers aspects like document control, change management, and deviations, fostering a culture of quality. Risk management and continuous improvement are also emphasized, ensuring product safety and efficacy. The manual serves as a guide for employees, auditors, and regulatory bodies, promoting transparency and accountability across all operations.
Click the link for part 1 of 2
https://pharmaguidehub.com/quality-manual-part-1-of-2/
Key Parameter of Below Page:
Resource Management
Find the below page content

Key Parameter of Below Page:
Resource Management
Find the below page content
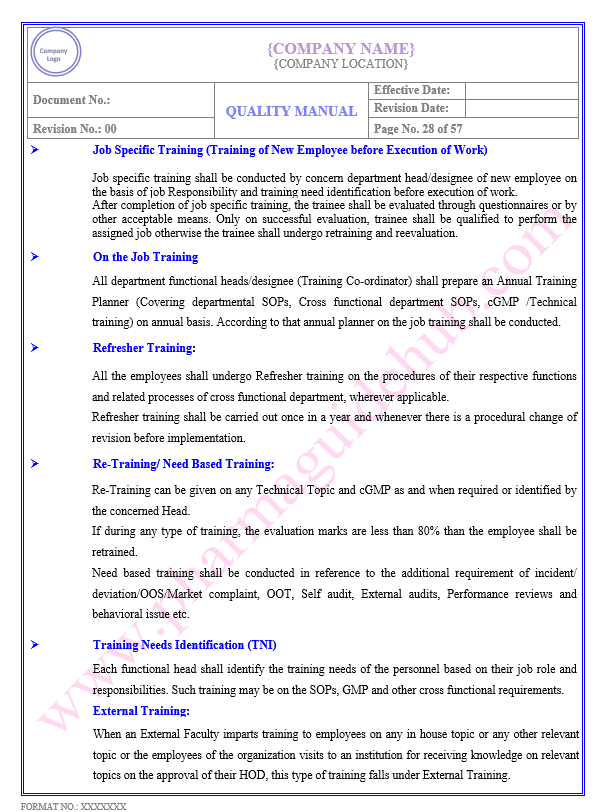
Key Parameter of Below Page:
Resource Management
Infrastructure
Find the below page content
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quality-manual/

Key Parameter of Below Page:
Vendor Evalution
Find the below page content

Key Parameter of Below Page:
Validation / Qualification Policy
Find the below page content

Key Parameter of Below Page:
Validation / Qualification Policy
Find the below page content
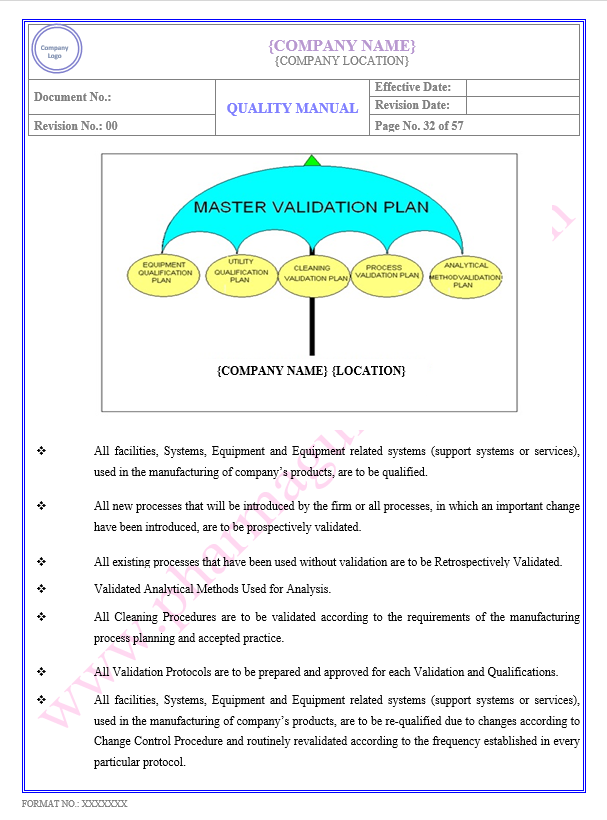
Key Parameter of Below Page:
Validation/Qualification Policy
Validation Program
Find the below page content

Key Parameter of Below Page:
Validation Program
Find the below page content

Key Parameter of Below Page:
Validation Program
Find the below page content
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quality-manual/
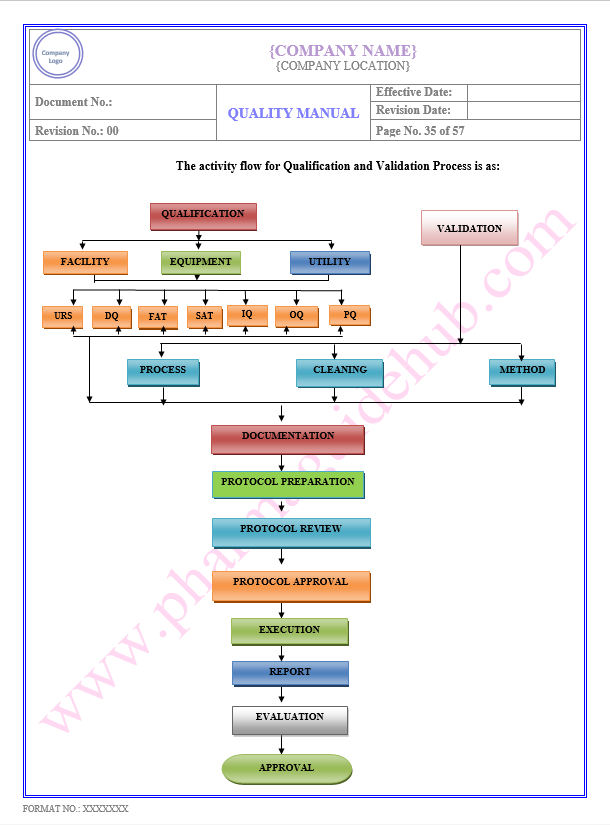
Key Parameter of Below Page:
Type of Validation
Find the below page content

Key Parameter of Below Page:
Type of Validation
Find the below page content
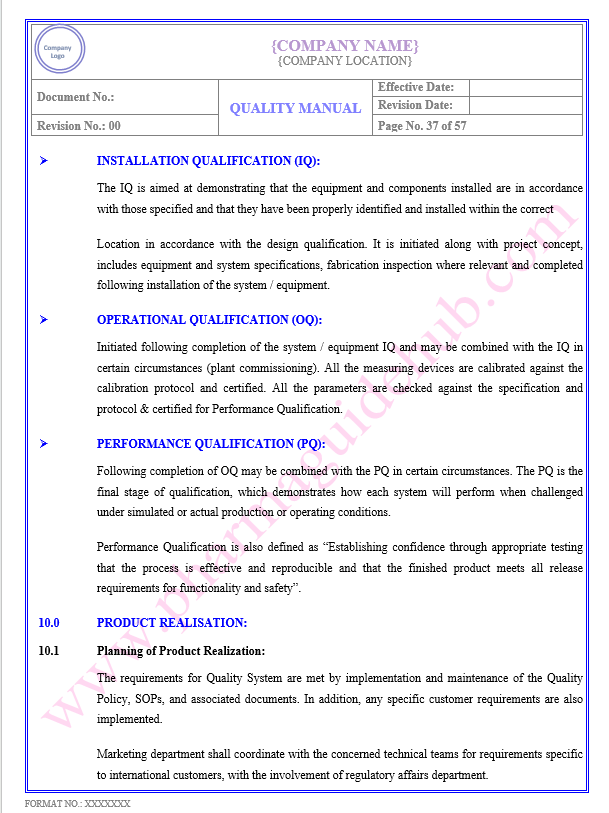
Key Parameter of Below Page:
Product Realisation
Planning of Product Realization
Find the below page content
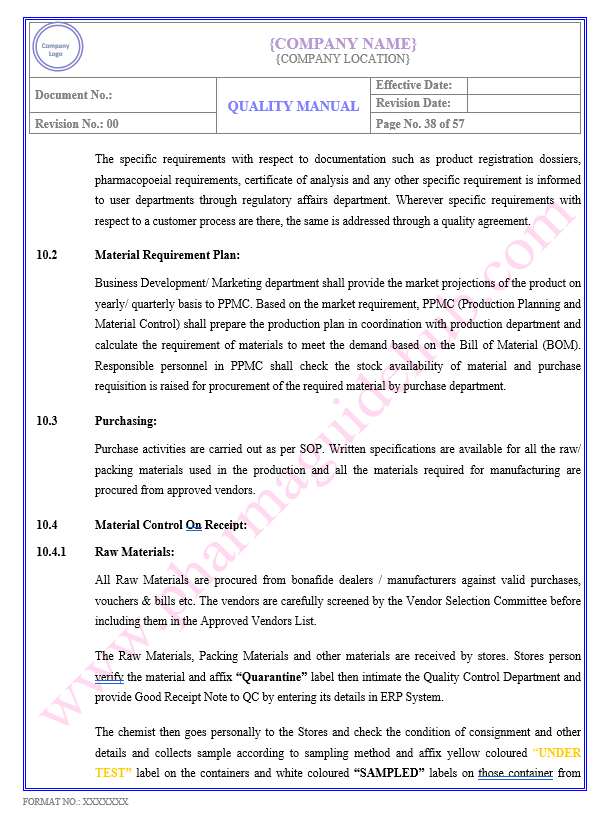
Key Parameter of Below Page:
Material Control On Receipt
Production Planning
Find the below page content
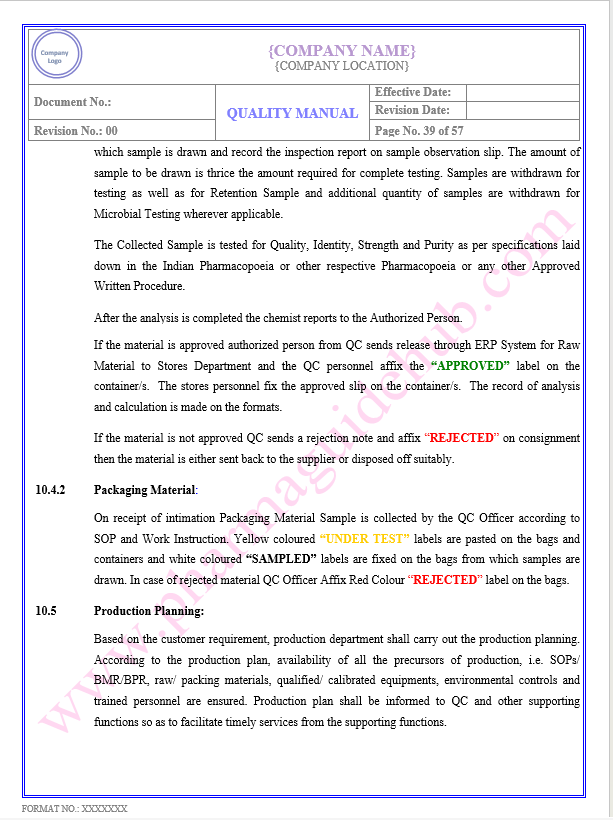
Key Parameter of Below Page:
Method, Process And Cleaning Validation
PRODUCTION/ PROCESS CONTROL
Find the below page content

Key Parameter of Below Page:
PRODUCTION/ PROCESS CONTROL
Find the below page content
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quality-manual/
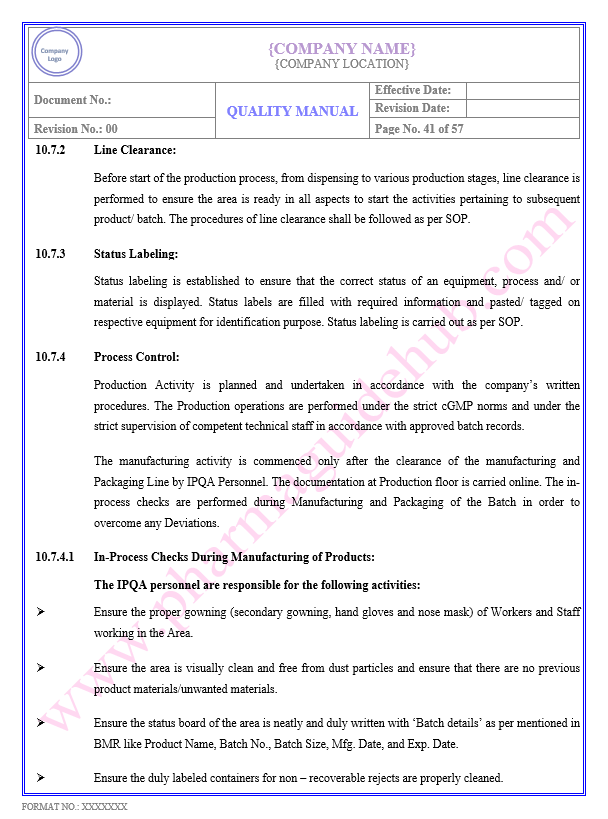
Key Parameter of Below Page:
PRODUCTION/ PROCESS CONTROL
Find the below page content
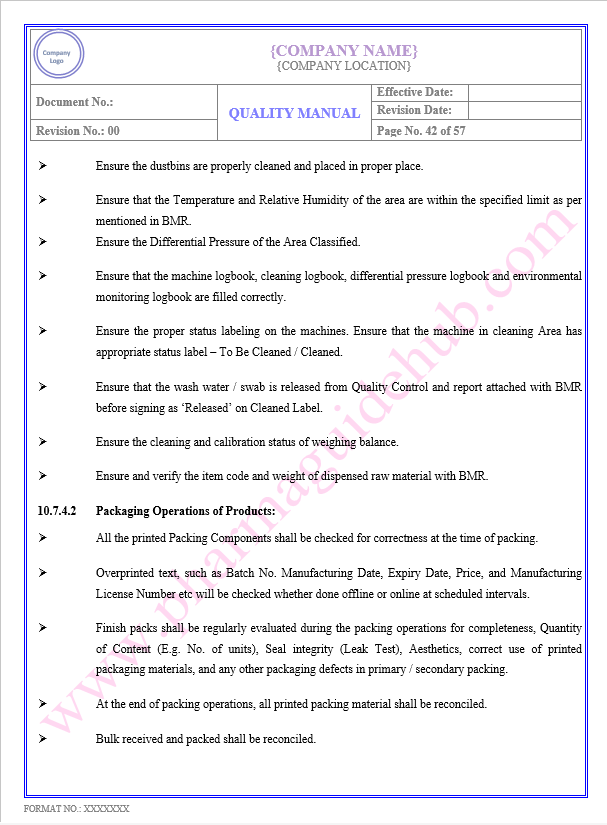
Key Parameter of Below Page:
PRODUCTION/ PROCESS CONTROL
Find the below page content
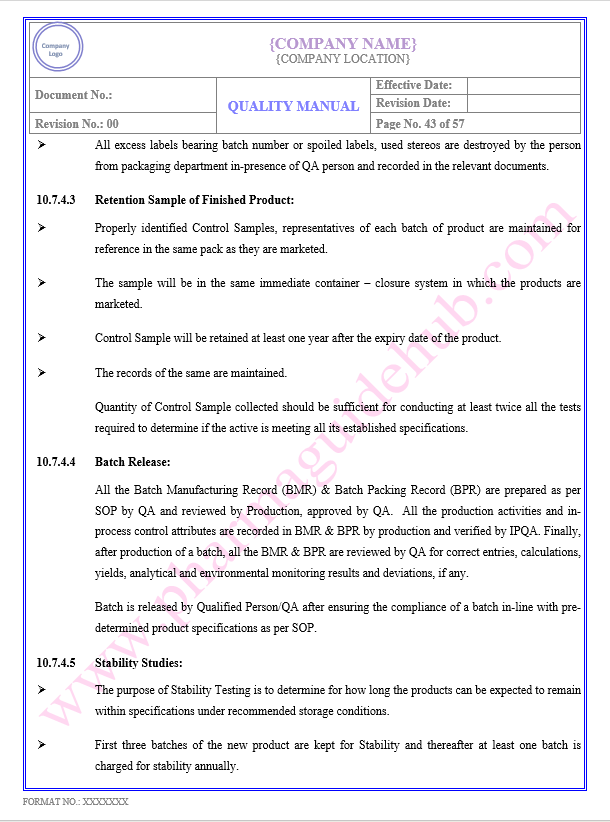
Key Parameter of Below Page:
PRODUCTION/ PROCESS CONTROL
Find the below page content

Key Parameter of Below Page:
PRODUCTION/ PROCESS CONTROL
Find the below page content
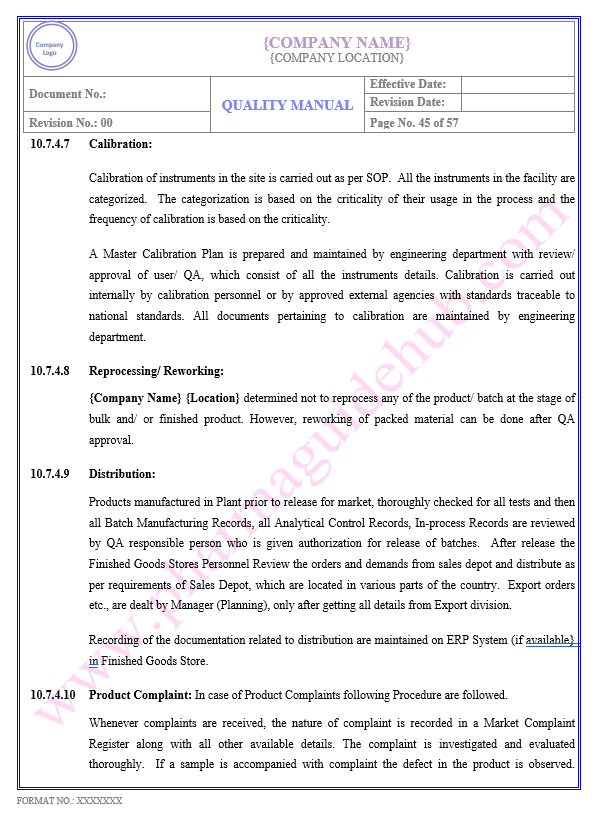
Key Parameter of Below Page:
PRODUCTION/ PROCESS CONTROL
Find the below page content

Key Parameter of Below Page:
PRODUCTION/ PROCESS CONTROL
Find the below page content
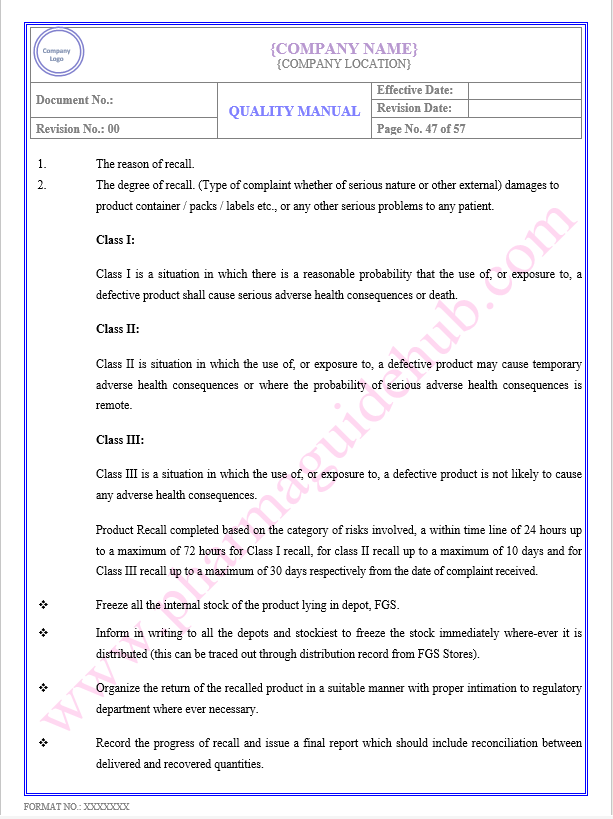
Key Parameter of Below Page:
PRODUCTION/ PROCESS CONTROL
Measurement, Analysis and Improvement
Find the below page content

Key Parameter of Below Page:
Measurement, Analysis and Improvement
Find the below page content
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quality-manual/
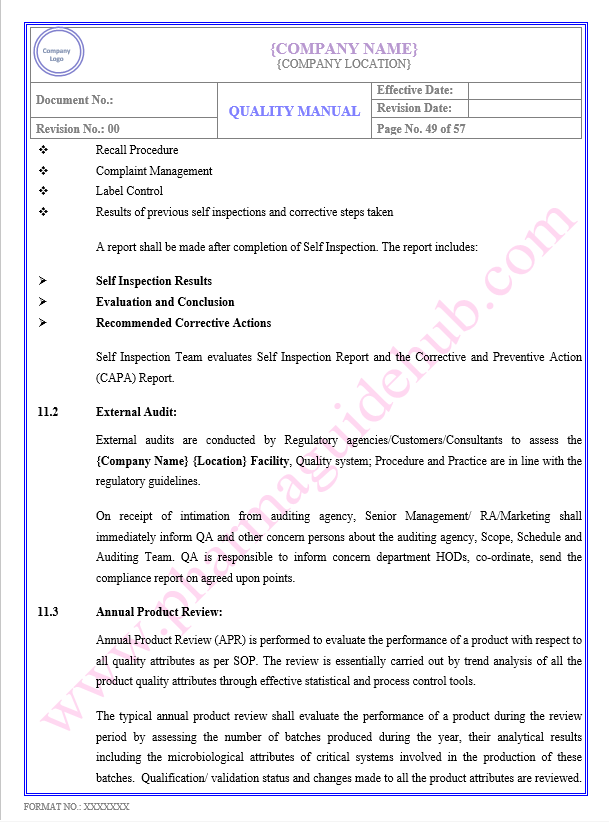
Key Parameter of Below Page:
Measurement, Analysis and Improvement
Water System
Referance
Find the below page content
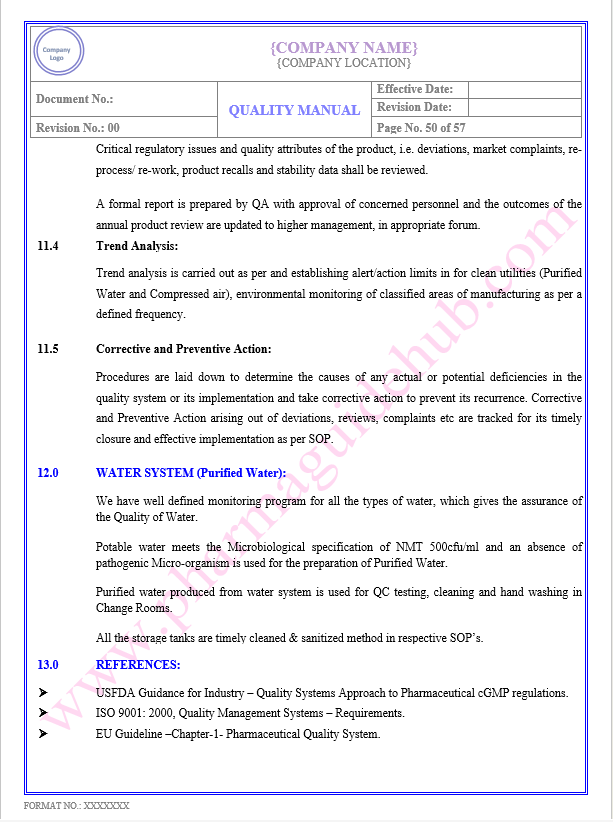
Key Parameter of Below Page:
Appendix
Find the below page content
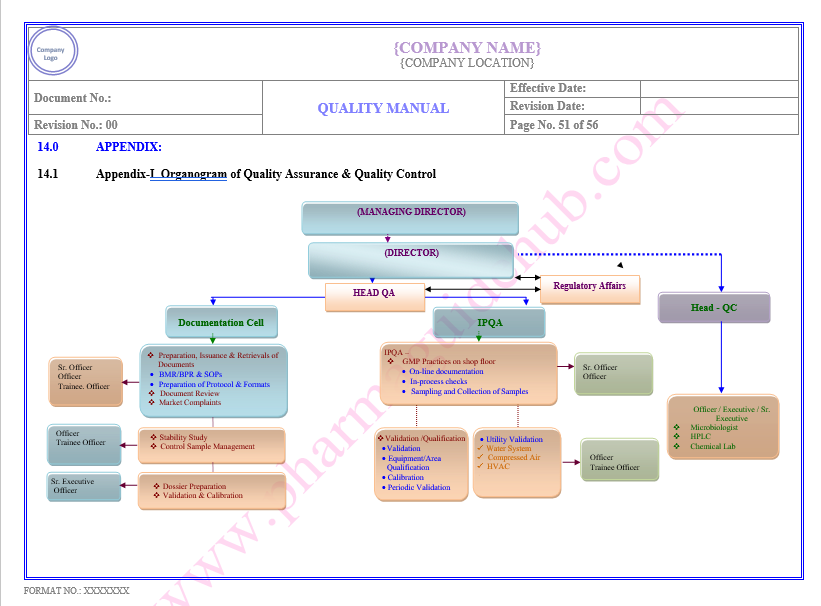
Key Parameter of Below Page:
Appendix
Find the below page content
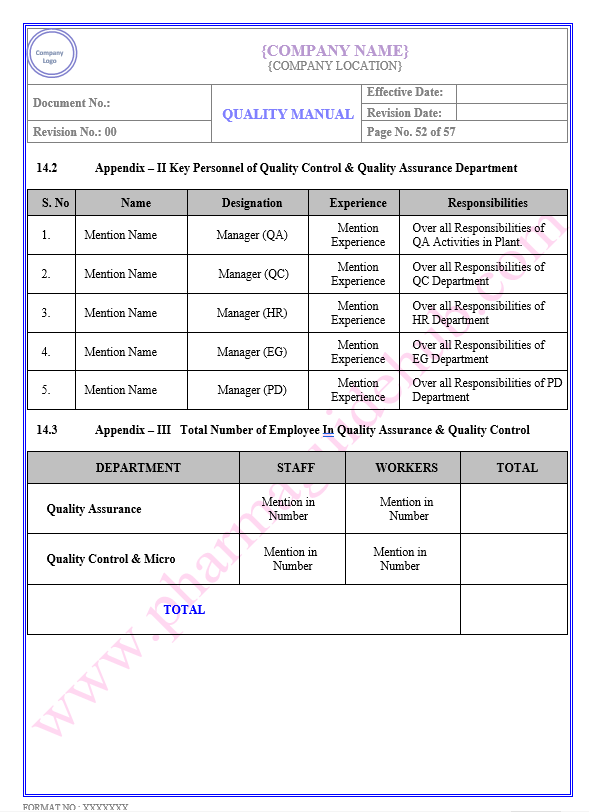
Key Parameter of Below Page:
Appendix
Find the below page content

Key Parameter of Below Page:
Appendix
Find the below page content
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quality-manual/

Key Parameter of Below Page:
Appendix
Find the below page content

Key Parameter of Below Page:
Appendix
Find the below page content

Key Parameter of Below Page:
Revision History
Find the below page content
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quality-manual/