- OBJECTIVE:
- To lay down the procedure for redressing of raw materials and packaging materials in Warehouse
- SCOPE:
- This SOP is applicable for the warehouse department at {Company Name} {Location}.
- RESPONSIBILITY:
- WH Executive/Designee – shall be responsible to follow the procedure as per SOP.
- Executive – QA shall be responsible for supervision of redressing activity.
- Head Warehouse – is responsible for compliance of the SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- Procedure for redressing of materials during receipt:
- During receipt of materials if any container is observed with damage/dent, the same shall be informed to QA with remarks in the receipt check list. Based on the QA comment, material redressing shall be performed if required.
- In case if any material received with damage of inner container/poly bags, and there is no material spillage observed inside the container, in such cases the inner container/poly bags shall be transferred to a fresh HDPE container under LAF of the sampling area/dispensing area in presence of IPQA personnel and affix the label and in such case the respective log book entry shall be done accordingly with remarks in the receipt check list.
- During receipt of material, if any vendor label found in damaged, torn or peel off condition, the same shall be informed to QA & purchase department accordingly and purchase department shall arrange the new labels from the supplier/vendor.
- Purchase department through mail and in such cases MRR shall be prepared after getting the new labels from the supplier/vendor.
- During receipt if any material received with damage/wet/torn and there is no impact on the materials, in such cases the materials shall be transferred to another fresh plain and labels shall be affixed on the fresh container with signature of warehouse and IPQA personnel.
- Transferring of all raw material and all primary packing materials to another fresh container shall be performed under LAF in sampling or dispensing area only.
- Any secondary packing materials shall be transfer to another fresh container / boxes / shipper in the secondary packing materials sampling or dispensing area only.
- In case of materials transferred from one code to another code, the required quantity shall be transferred from original container/pack to suitable fresh container and label shall be affixed as per the Format-II.
- Procedure for redressing during storage and handling of raw and packaging materials:
- After receipt and during sampling/storage/handling of materials, if any outer container / shipper is found in damaged condition, the same shall be replaced with a suitable fresh container/ shipper by warehouse personnel under the supervision of IPQA personnel and affix the Redressed material labels (“Transferred Material “label) as per Format-II on the container and the same shall be intimated to QC.
- During sampling/storage/handling of any materials, found with any small dent/damage on the outer side of the container/shippers and there is no damage of inner container or material, then the outer container/shipper shall be covered with fresh double lined poly bags and shall be closed with cable tie and affix the redressed label.
- During sampling/storage/handling of any materials if any damage or spillage of material is observed form the container, immediately inform to IPQA personnel and section in charge, arrest the spillage immediately and segregate the spilled material container and transfer to the rejected material storage area and proceed further as per the QA instruction.
- During sampling/storage/handling of any secondary packing materials outer container/inner container damaged, the same shall be redressed during pack damaged, effected package shall be transfer to secondary packing materials dispensing area and transfer the secondary into plain shipper, paste the label as per the format-II.
- Procedure for Redressing/repacking of raw materials and packaging materials received from production through MRN:
- RAW MATERIALS:
- If any dispensed raw materials are returned to warehouse through MRN the same shall be handled as underAPI shall be repacked in fresh and suitable HDPE containers or shippers as per the applicability along with the dispensed label (material issue tag) inside and one computer generated label shall be affixed on outer side of the container.
- If any API is returned to warehouse and the individual pack quantity is not able to accommodate in one single container then the same shall be packed in multiple containers.
- In such cases, the dispensed material label (material issue tag) shall be put inside of any one container and for all other containers, one label as per Format-II shall be placed inside the container and one label shall be affixed on the outer side of the container/shipper for the identification of material.
- Transfer the materials in designated area as per the storage condition.If any inactive materials returned to store after dispensing, the same shall be repacked in separate suitable container and if one individual pack is not able to accommodate in one single container then the same shall be packed in multiple containers and label.
- In case if any solvent is returned to stores through MRN, the same shall be kept separately in suitable container with status label.
- If any MRN material is available in stock, the same shall be dispensed first as per the requirement of production.
- Repacking of all inactive materials and all primary packaging materials shall be performed under LAF in dispensing area only and the repacking activity shall be performed in presence of IPQA personnel.
- PACKAGING MATERIALS:
- All primary packaging materials shall be repacked in fresh and suitable shipper as per the applicability along with the dispensed label (material issue tag) inside and one label shall be affixed on outer side of the container.
- For all secondary and tertiary packaging materials shall be kept in original containers if space available and if space is not available in the original/mother containers/shippers, then the same shall be packed in separate and suitable containers/shippers along with the dispensed label (material issue tag) inside the container and one label shall be affixed on the outer side of the container/shipper and transfer the materials in designated area.
- If any MRN material is available in stock, the same shall be dispensed first as per the requirement of production/packing.
Note: In case of quarantine/approved peeled off or shade variation during handling and storage of materials in the warehouse, the same shall be intimate to QC/QA for new label. Paste the new label in the presence of IPQA.
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Intimation for Redressing of Raw and Packaging Materials |
| Annexure-II | Transferred label |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Warehouse
- Controlled Copy No. 03 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| PGH | : | Pharmaguidehub |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| API | : | Active Pharmaceutical Ingredients |
| QA | : | Quality Assurance |
| MRR | : | Material Received Report |
| COA | : | Certificate Of Analysis |
| HDPE | : | High Density Polyethylene |
| NCR | : | Non Conformance Report |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
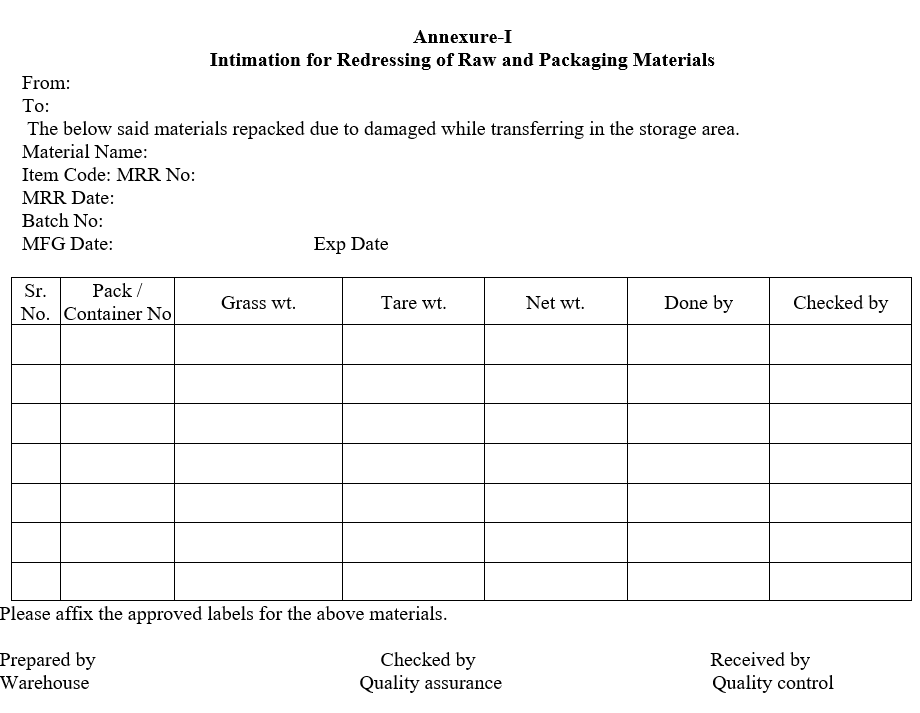
Annexure-I
Intimation for Redressing of Raw and Packaging Materials
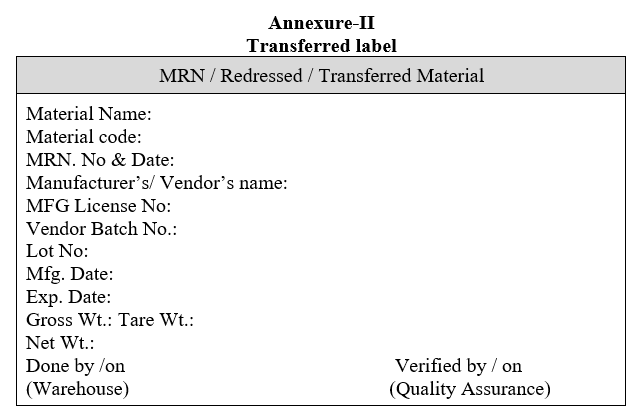
Annexure-II
Transferred label
Frequently Asked Question?
1. What happens if a container arrives damaged during receipt?
Answer: If a container is damaged upon arrival, inform QA and note the damage in the receipt checklist. Depending on the severity, QA will decide if the material needs redressing.
2. How do you handle damaged inner containers without spillage?
Answer: For damaged inner containers without spillage, transfer the contents to a fresh HDPE container under LAF in the presence of IPQA personnel. Affix a new label and document the process in the logbook.
3. What to do if vendor labels are damaged?
Answer: Inform QA and the purchase department if vendor labels are damaged. The purchase department will obtain new labels from the supplier, and an MRR will be prepared after receiving them.
4. How do you handle slightly damaged materials without impacting the product?
Answer: For slightly damaged materials without product impact, transfer them to a fresh container with new labels. This process must occur under LAF in the designated sampling or dispensing area.
5. What happens if materials need to be transferred from one container to another?
Answer: When transferring materials, ensure the correct quantity goes into a suitable fresh container. Label the new container according to Format-II.
6. How do you address damaged containers during storage and handling?
Answer: Replace damaged outer containers with fresh ones under IPQA supervision. Affix a “Transferred Material” label and inform QC.
7. What to do for minor dents on outer containers without inner damage?
Answer: Cover minor dents with fresh double-lined polybags, secure them with cable ties, and affix a redressed label.
8. How do you handle material spillage during storage and handling?
Answer: Immediately inform IPQA and the section in charge. Contain the spillage, segregate the affected container, and move it to the rejected material storage area. Follow further instructions from QA.
9. How do you repackage secondary packing materials with damaged containers?
Answer: Transfer the damaged secondary packing materials to the dispensing area and repack them into plain shippers with labels following Format-II.
10. How do you handle raw materials and packaging materials returned from production?
Answer: Repack returned materials in suitable containers with appropriate labeling procedures. Ensure proper storage based on material type.
11. What happens if quarantine/approved labels peel off or experience shade variations?
Answer: Inform QC/QA for new labels and replace them in the presence of IPQA personnel.
