- PROCEDURE:
- The types of samples are:
- Raw Material Samples
- In process Samples
- Finished Product Samples
- Packing Material Samples
- Working Standard Samples
- Registration shall be also done for below samples as:
- Method Transfer Samples (Raw Material and Finished Product).
- Process validation Samples
- Hold Time Study Samples
- Cleaning validation samples
- Equipment Validation Samples
- Vendor SamplesProduction
- Aid Samples Innovator Samples
- Process Development Samples
- Stability Samples
- Miscellaneous Samples
- Sample Registration system:
- After receiving the samples Section Head or Section In charge is responsible to allocate the samples.
- Method transfer samples shall be registered as per SOP.
- Raw Material samples shall be registered as per SOP
- In Process Samples shall be registered as per SOP
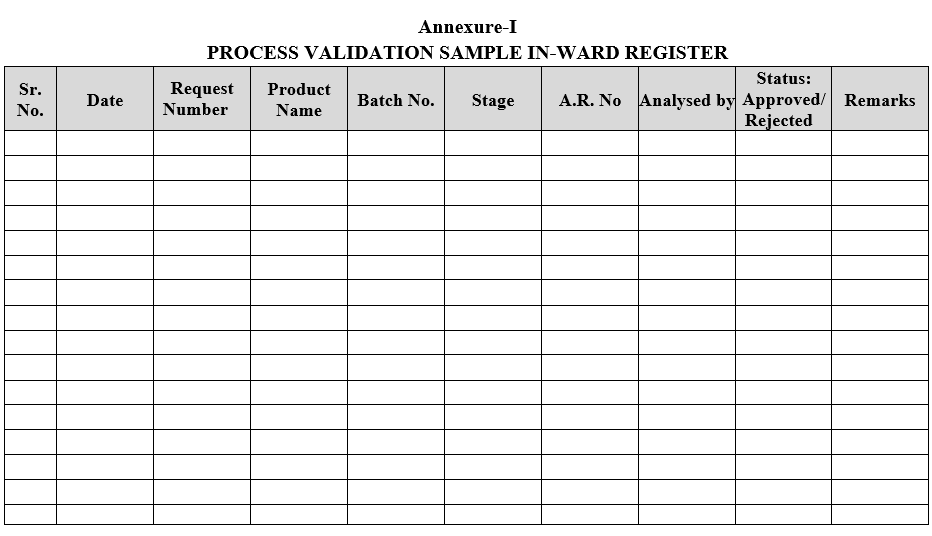
- Process Validation samples shall be registered as per Format-I.
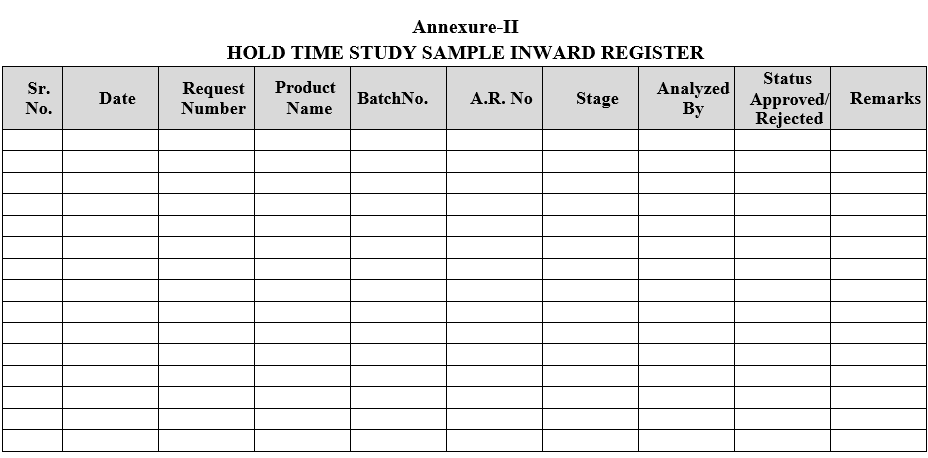
- Hold Time Study samples shall be registered as per Format-II.
- Finished Product, working standard and packing material samples shall be registered as per SOP.
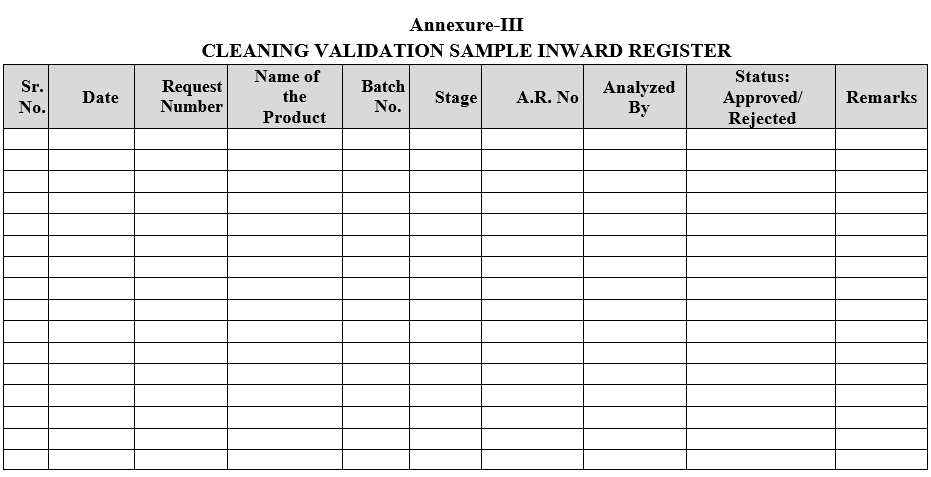
- Cleaning Validation samples shall be registered as per Format-III.
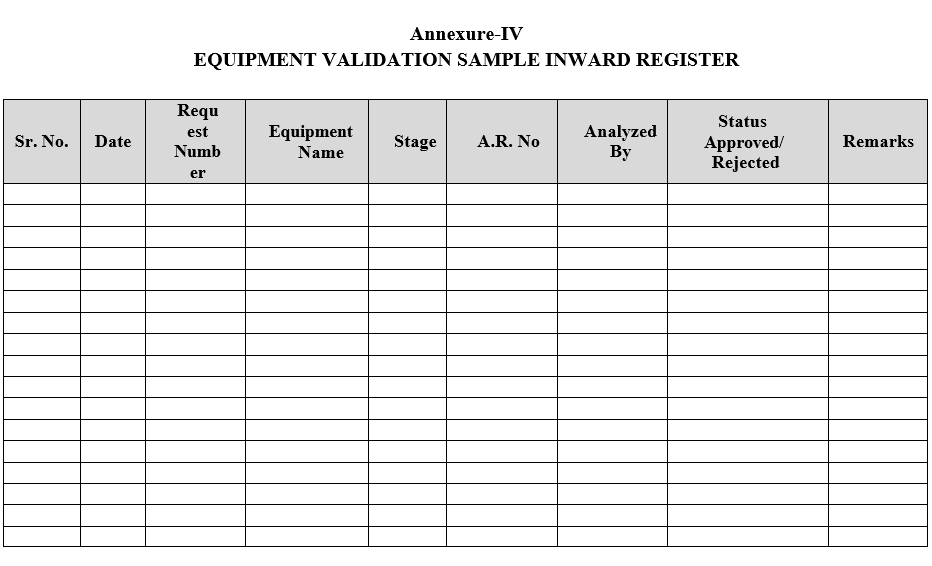
- Equipment Validation samples shall be registered as per Format-IV.
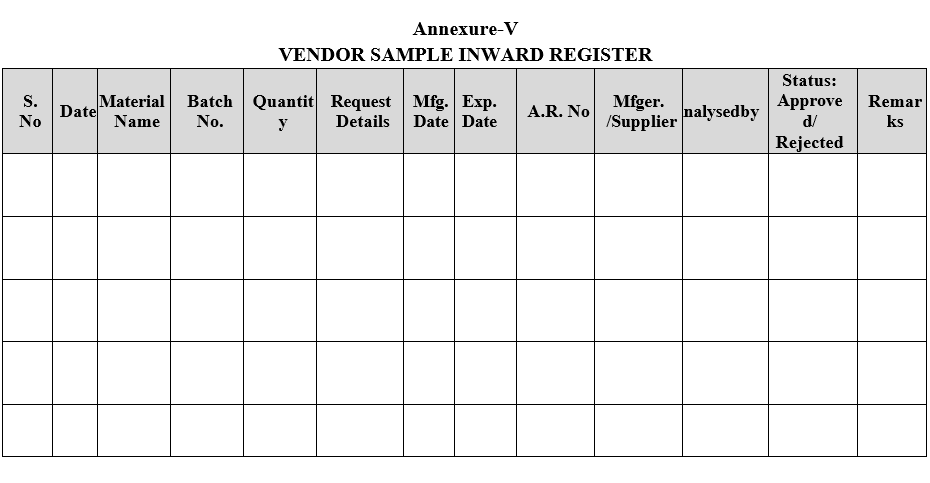
- Vendor samples shall be registered as per Format-V.
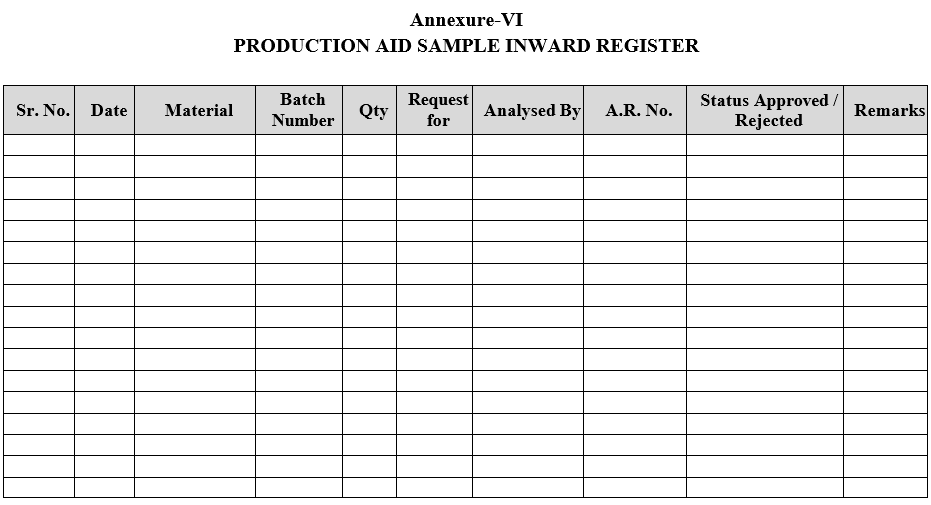
- Production Aid Samples shall be registered as per Format-VI.
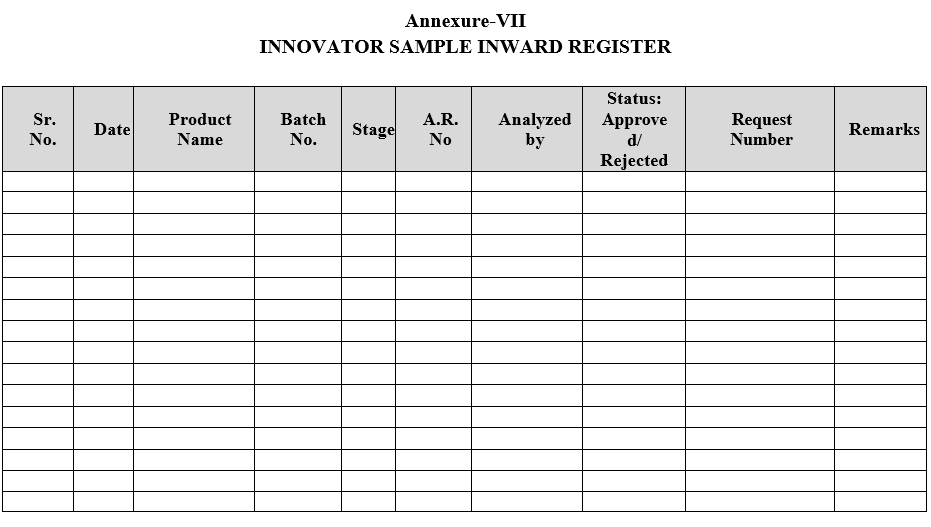
- Innovator samples shall be registered as per Format-VII.
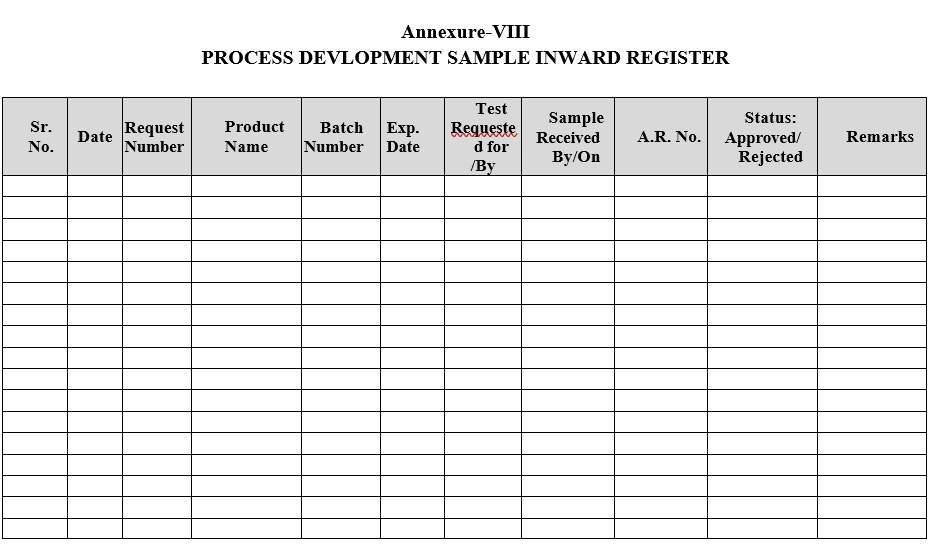
- Process Development samples shall be registered as per Format-VIII.
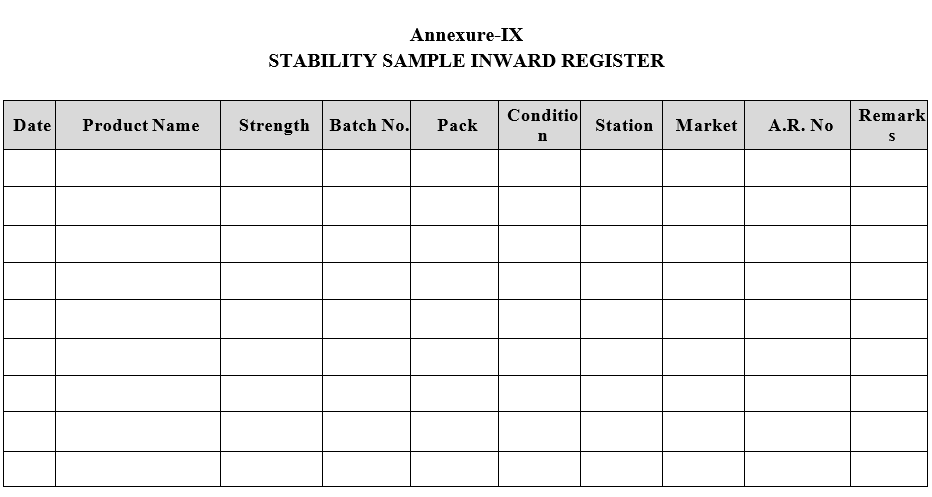
- Stability Samples shall be registered as per Format-IX.
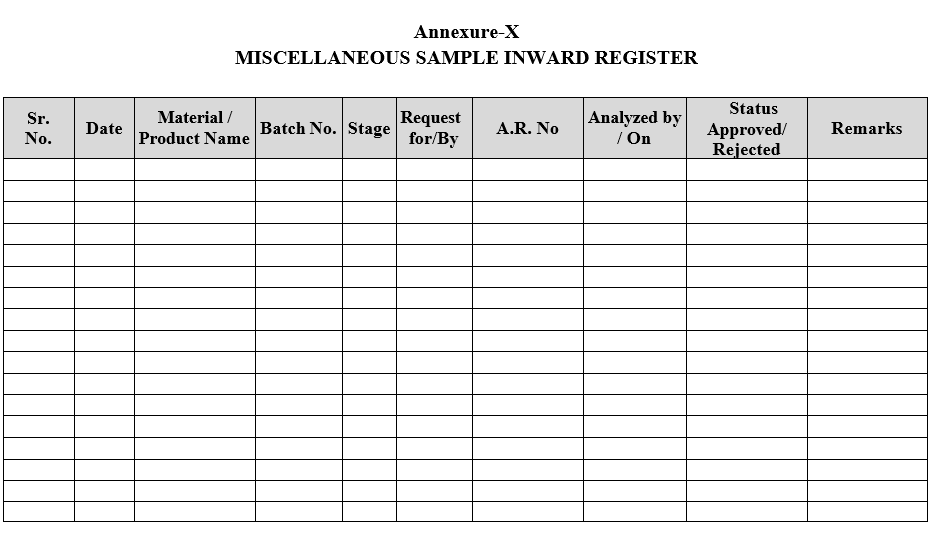
- Miscellaneous samples shall be registered as per Format-X.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Process Validation Sample In-ward Register. |
| Annexure-II | Hold Time Study Sample In-ward Register |
| Annexure-III | Cleaning Validation Sample In-ward Register |
| Annexure-IV | Equipment Validation Sample In-ward Register |
| Annexure-V | Vendor Sample In-ward Register |
| Annexure-VI | Production Aid Sample In-ward Register |
| Annexure-VII | Innovator Sample In-ward Register |
| Annexure-VIII | Process Development Sample In-ward Register |
| Annexure-IX | Stability Sample In-ward Register |
| Annexure-X | Miscellaneous Sample In-ward Register |
Annexure-I
PROCESS VALIDATION SAMPLE IN-WARD REGISTER
Annexure-II
HOLD TIME STUDY SAMPLE INWARD REGISTER
Annexure-III
CLEANING VALIDATION SAMPLE INWARD REGISTER
Annexure-IV
EQUIPMENT VALIDATION SAMPLE INWARD REGISTER
Annexure-V
VENDOR SAMPLE INWARD REGISTER
Annexure-VI
PRODUCTION AID SAMPLE INWARD REGISTER
Annexure-VII
INNOVATOR SAMPLE INWARD REGISTER
Annexure-VIII
PROCESS DEVLOPMENT SAMPLE INWARD REGISTER
Annexure-IX
STABILITY SAMPLE INWARD REGISTER
Annexure-X
MISCELLANEOUS SAMPLE INWARD REGISTER