- OBJECTIVE
To lay down a procedure Releases of Batches for Packing and Sale.
- SCOPE
This SOP is applicable for Releases of Batches for Packing and Sale at {Company Name} {Company Location}.
- RESPONSIBILITY
- Executive/Manager Production/Packaging shall be responsible for reviewing of BMR/BPR before submitted to IPQA/QA.
- Officer/Executive/Manager QA shall be responsible for review of Batch records. Head QA/Designee and his authorized designee are responsible for release of batch for dispatch from site.
- ACCOUNTABILITY
Head -Quality Assurance
- PROCEDURE
- In the pharmaceutical industry, batch release refers to a critical process that ensures the quality and safety of medications before they are packaged and distributed for sale. It’s a multi-step procedure governed by strict regulations to comply with Good Manufacturing Practices (GMP).
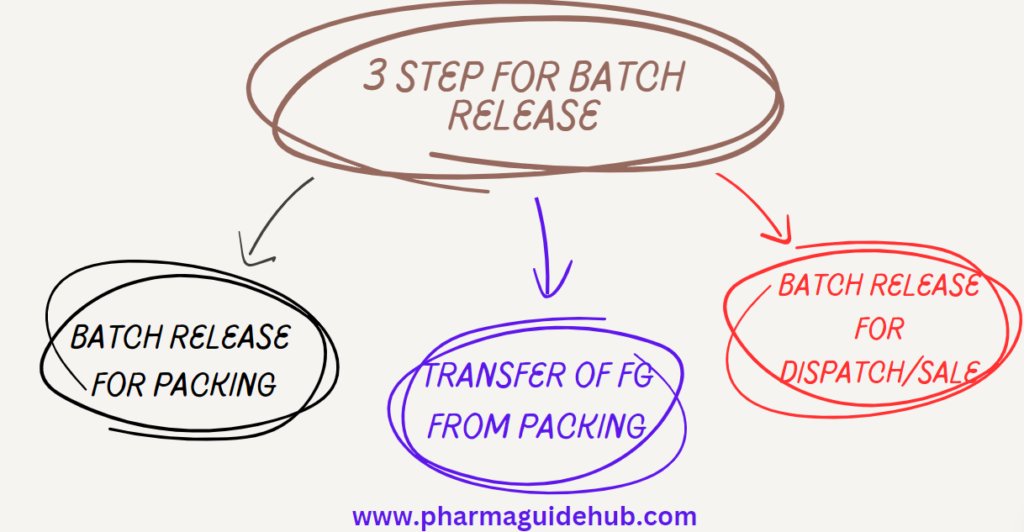
- Batch Release for Packing
- Following documents shall be reviewed by QA personnel before release for packaging.
- BMR shall be thoroughly reviewed by designated IPQA personnel for each batch with focus on following attributes as per SOP.
- Current version of BMR is being used & Batch numbering SOP is being followed as per product specific requirement.
- Correctness of Shelf life, Manufacturing Date and Expiry Date of product need to be verified. In case of domestic product, Revised Schedule M guidelines requires assigned shelf life of product shall not exceed available shelf life of any of the API used in particular batch of product.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/release-of-batches-for-packing-and-sale/
- Stage wise Line Clearance of each area as per respective SOP.
- Environmental monitoring records need to be in line as specified in BMR.
- Effective implementation of Good Documentation as per SOP.
- Appropriate closure of Deviation / Investigation for deviation or incidents if any as per respective SOP.
- Bill of Material vs Actual Quantity Dispensed as per BMR/ dispensing slip. Correctness of AR. No of each material. Specific attention to be given to quantity of API used and its calculation for compensation of water /assay if applicable.
- Stage wise IPQA checks, production checks, yield reconciliation, printout of weighing records & equipment printout where applicable.
- Release slip from QC for rinse water analysis if applicable for equipment / area clearance & stage wise release for next stage based on predefined specifications at each stage. Based on product specific trend data and as per approved documents IPQA officers are authorized to release batch to be processed for next stage.
- All CCPs and CQA for each stage are within specified limits in BMR.
- Batch to be released for Packing need to comply with AQL SOP.
- Based on satisfactory review of above stated points, checklist need to be filled by designated QA personnel in BMR to effect release for packaging. Subsequently IPQA officer shall affix ‘APPROVED’ label on each container. Labels pasted on each container shall be verified for correctness with respect to Product Name, Batch Number, Mfg./ Expiry Date, Weight, and total number of containers per batch prior to affixing Approved labels.
- All containers of particular batch of product having “APPROVED” labels are transferred into Ready for Packing Area.
- Packaging department/IPQA shall follow respective SOP further to initiate packaging operation of particular batch of Product.
- Transfer of FG from Packing
- Once Batch of Product is packed and is ready for transfer from Packing Hall/ FG Day Store to FG Warehouse designated QA person need to review BPR with focus on following attributes as per the SOP
- Once Batch of Product is packed and is ready for transfer from Packing Hall/ FG Day Store to FG Warehouse designated QA person need to review BPR with focus on following attributes as per the SOP.
- Record of release of batch for packaging in BMR.
- Current version of BPR is being used & Batch numbering SOP is being followed as per product specific requirement.
- Correctness of Shelf life, Manufacturing Date and Expiry Date of product need to be verified. It has to match with BMR/ requisition slips. In case of domestic product, MRP of product as printed on product need to be verified for correctness. If there is change in MRP, effective from batch under review client communication need to be verified and its record need to be attached in BPR. Overprinting details on product need to be checked for legibility, fonts or any other client/regulatory specific requirement mentioned in BMR or client communication file.
- Records of Dispensing of Packaging Material need to be correct in terms of Material Code/Artwork number if applicable/ AR. No. as mentioned in BPR & requisition / Issuance, Return & Reject records of material as recorded in BPR & Issuance, print proofs & Destruction of Stereos /review of Print proofs attached in BPR/ Pack configuration & Pack style/physical review of one packed unit.
- Records of Line Clearance of each area/equipment as per respective SOP.
- Effective implementation of Good Documentation as per SOP.
- Records of Appropriate closure of Deviation / Investigation for incidents if any as per respective SOP.
- Records of IPQA checks, packaging checks, yield reconciliation, printout of weighing records & equipment printout where applicable.
- Release slip from QC for rinse water analysis if applicable for equipment / area clearance. Record of sampling/submission of specified quantity of packs of products for analysis to QC / Retention/ Control Samples/ Stability samples etc as applicable.
- All CCPs and CQA for packing stage are within specified limits in BMR.
- Batch to be released for transfer of goods to FG warehouse need to comply with SOP of Packed stock inspection.
- Based on satisfactory review of above stated points, checklist need to be filled by designated QA personnel in BPR to effect transfer of finished goods to FG Warehouse. Subsequently packaging officer and warehouse officer need to transfer Finished Goods to FG Warehouse.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/release-of-batches-for-packing-and-sale/
- Batch Release for Dispatch/Sale:
- Batch release for dispatch/sale shall be approved by Head QA/Designee after compliances of following steps.
- Reviewed Batch Manufacturing Record shall be available with duly filled check list for release for packaging.
- Reviewed Batch Packing Record shall be available with duly filled check list for transfer of packed finished goods to FG Warehouse.
- Records of review and availability of Analytical raw data by QC along with COA. COA shall be reviewed to ensure results are as per current specification current standard testing procedures are followed.
- Records of incident/ deviations/CAPA and handling/closure of same as per SOP.
- After review of the BMR/BPR the batch shall be released by the Head Quality Assurance or his authorized Designee who shall sign on the ‘Batch Release Order as per Annexure –II.
- Finally, the ‘Batch Release Order’ shall be sent to the Warehouse as intimation for the batch release for sale and distribution. A copy of the Batch Release Order is attached in the Batch production record.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/release-of-batches-for-packing-and-sale/
- REFERENCES:
Not Applicable.
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
| Annexure-I | BPR review Checklist |
| Annexure-II | Batch Release Order |
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Controlled Copy No. 03 : Head Production
- Controlled Copy No. 04 : Head Warehouse
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| SOP | : | Standard Operating Procedure |
| QA IPQA | : : | Quality Assurance In-Process Quality Assurance |
| QC | : | Quality Control |
| OOS | : | Out of Specification |
| RH | : | Relative Humidity |
| BMR | : | Batch Manufacturing Record |
| BPR MFG. EXP. | : : : | Batch Packing Record Manufacturing Expiry |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
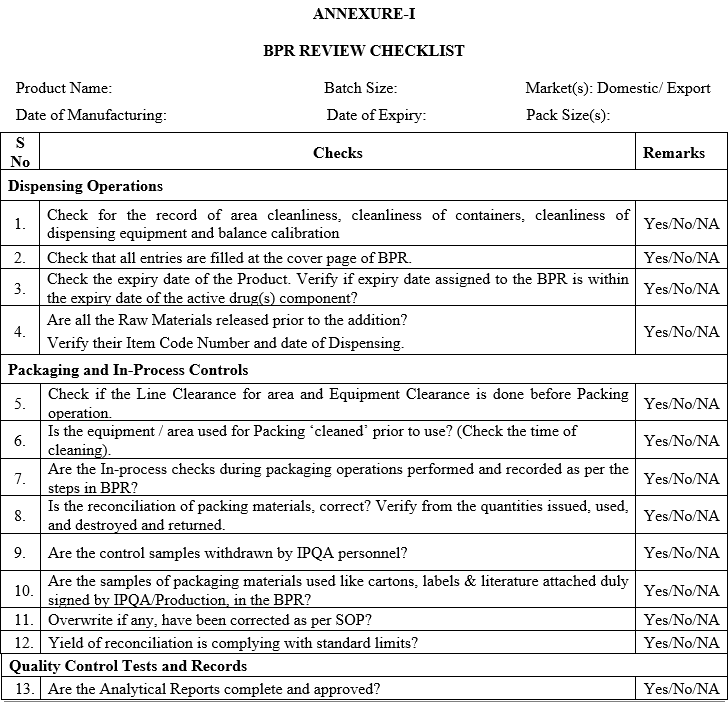
ANNEXURE-I
BPR REVIEW CHECKLIST

ANNEXURE-II
BATCH RELEASE ORDER

Click the link for download word file copy of this document: https://pharmaguidehub.com/product/release-of-batches-for-packing-and-sale/
Frequently Asked Question ?
- What is the overall purpose of this document?
This document outlines the procedures for releasing batches of finished goods for various stages of production, including packing, transfer to the warehouse, and final dispatch/sale.
2. Who is this document intended for?
This document is likely intended for quality assurance (QA) personnel involved in the manufacturing and release of finished goods.
3. What documents are reviewed by QA personnel before releasing a batch for packing?
Several documents are reviewed, including the Batch Manufacturing Record (BMR), Batch Packaging Record (BPR), and various quality control checks and environmental monitoring records.
4. What specific aspects of the BMR are reviewed?
The BMR is reviewed for adherence to relevant SOPs, correct shelf life and expiry dates, appropriate closure of deviations, and accurate bill of materials and dispensing details.
5. What criteria must be met for a batch to be released for packing?
All CCPs and CQAs must be within specified limits, the AQL SOP must be followed, and all containers must be labeled correctly and approved by QA personnel.
6. What does the QA person review in the BPR before transferring the batch from packing?
The QA person reviews the BPR for documentation of batch release for packaging, proper use of BPR versions and batch numbering, correct shelf life and expiry dates, and accurate records of packaging material dispensing and line clearance.
7. What additional checks are performed before transferring the goods to the warehouse?
Records of IPQA checks, reconciliation, print proofs, and destruction of stereos are reviewed, along with packaging configuration, pack style, and physical inspection of a packed unit.
8. How is the transfer of finished goods to the warehouse authorized?
Upon satisfactory review of all required documents and checks, the designated QA personnel fills out a checklist in the BPR and, along with the packaging officer, transfers the goods to the warehouse.
9. Who approves the final batch release for dispatch/sale?
The Head QA or their designee approves the final release after reviewing the completed BMR and BPR, analytical raw data with Certificate of Analysis (COA), incident handling/closure records, and signing the Batch Release Order.
10. What does the Batch Release Order signify?
The Batch Release Order serves as official authorization for the warehouse to release the batch for sale and distribution.
11. Where is the Batch Release Order kept?
A copy of the Batch Release Order is attached to the Batch Production Record for documentation purposes.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/release-of-batches-for-packing-and-sale/