- PROCEDURE:
- After completion of the analysis, the analytical chemist shall calculate the analytical result in the work sheet and compare it with the stated limits in the specification.
- The calculated analytical values shall be reported in the worksheets, COAs and raw data sheets as given in the following table:
Methodology for Reporting of Analytical Results:
* At the end of the COA mention the expansion of LOQ, LOD and Disregarding value.
- When reporting the results of the Related substances, see the disregarding limits of Unknown impurities and LOD & LOQ limits of known impurities. Do not consider the Unknown impurities which are below the disregarding limit.
- If the Known impurity is less than the LOD do not consider for calculation.
- If the Impurity value is more than LOD limit and less than the LOQ do not consider for calculating total impurities and individual impurities.
- Any Known impurity value more than the LOQ limit shall be considered for calculation of total impurities.
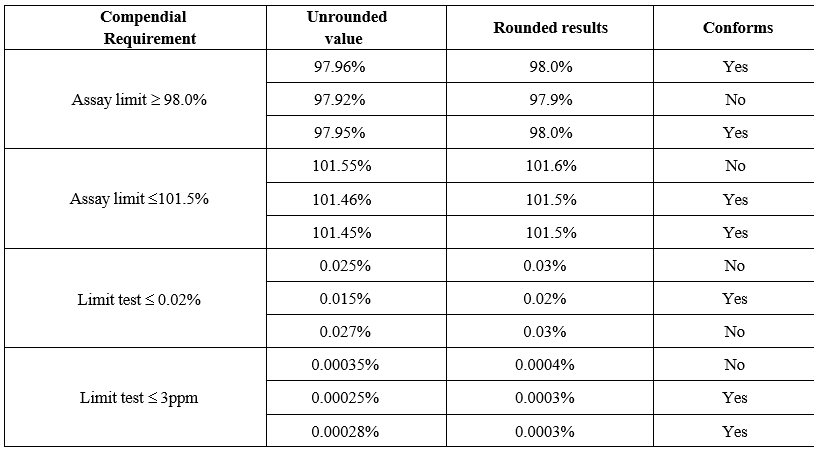
- If the calculated value(s) contains more digits in the decimal place than that in the stated limit, The digits after decimal place shall be rounded off as described below.
- Rounding off shall be done by considering only one digit in the decimal place to the right of the last place in the limit expression as shown in the illustration below
- If the digit is smaller than 5, it shall be eliminated and the preceding digit shall remain unchanged.
- If the digit is greater than or equal to 5, it shall be eliminated and the preceding digit shall be increased by one.
Note: Limits are fixed numbers and shall not be rounded off.
- Methodology for Rounding off Analytical Values:
| S. No. | Test | Parameter | Reporting method |
| 1. | Related Substances | More than 1.0% | One digit after the decimal |
| 2. | Related Substances | Less than or equal to 1.0% (1.0 % – 0.1%) | Two digits after the decimal |
| 3. | Related Substances | Less than or equal to 0.1% (0.1% – 0.01%) | As per LOQ decimal |
| 4. | Related Substances | Less than 0.01% (0.01% – 0.001%) | As per LOQ decimal |
| 5. | Dissolution | – | One digit after the decimal |
| 6. | Assay in mg | – | Above 100mg one digit after the decimal and Below that as per specification |
| 7. | Assay in % | – | One digit after the decimal |
| 8. | Content Uniformity in mg | – | Above 100mg one digit after the decimal and Below that as per specification |
| 9. | Content Uniformity in % | – | One digit after the decimal |
| 10 | Water content (By KF) , Particle Size (By Malvern), Viscosity DSC, Melting point and refractive index and Specific optical Rotation etc. | – | Report as per instrument output. |
Illustration of Rounding Numerical Values for comparison with requirements:
- When the analytical method or test requires quantification, in such cases, the analytical result shall be expressed as observed numerical value.
- When the analytical method or test does not require quantification, in such cases the analytical Result shall be expressed as “Conform” or “does not conform” as the case may be on Certificate of Analysis.
- However, in the work sheet, the actual observation of analysis shall be specified (for Example: Less than 50ppm, colour intensity of sample solution is not more than that of standard Solution “x” or formation of specific colour to the solution or formation of precipitate).
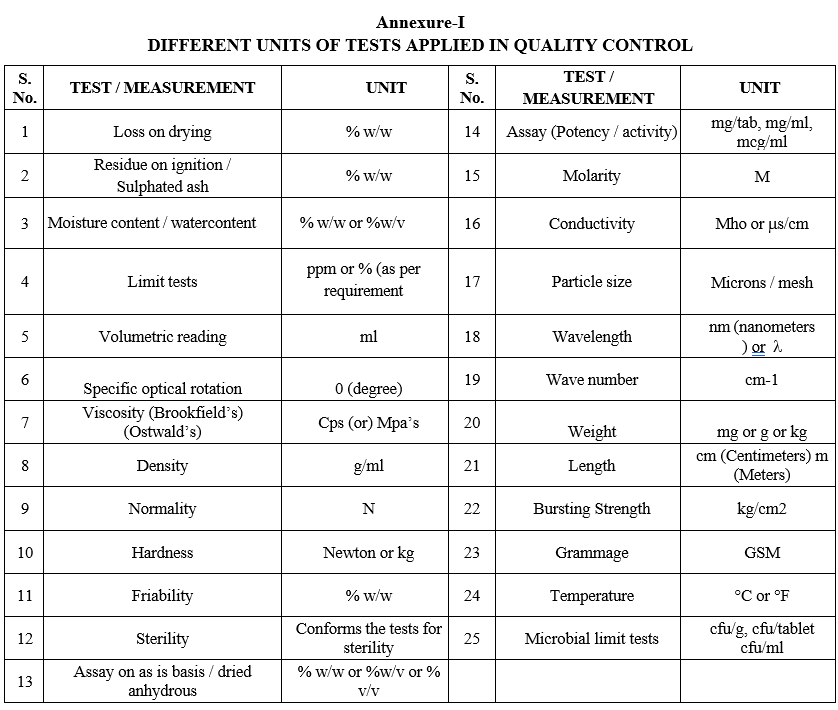
- Analytical values or results for the tests shall be expressed with units as given in Format-I.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Different Units of Tests applied in Quality Control |
- ABBREVIATIONS:
| No. | : | Number |
| LOD | : | Limit of Detection |
| LOQ | : | Limit of Quantification |
| COA | : | Certificate of Analysis |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
Annexure-I
DIFFERENT UNITS OF TESTS APPLIED IN QUALITY CONTROL