- OBJECTIVE:
The lay down a SOP is to describe the responsibilities of Quality Assurance Department.
- SCOPE:
This SOP is applicable to the Quality Assurance department at {Company Name} {Company Location}.
- RESPONSIBILITY:
QA Officer – Preparation of SOP.
QA Executive/designee Review and technical correction of SOP.
QA Head/designee –Training, approval & implementation of SOP.
- ACCOUNTABILITY:
Head QA
- PROCEDURE:
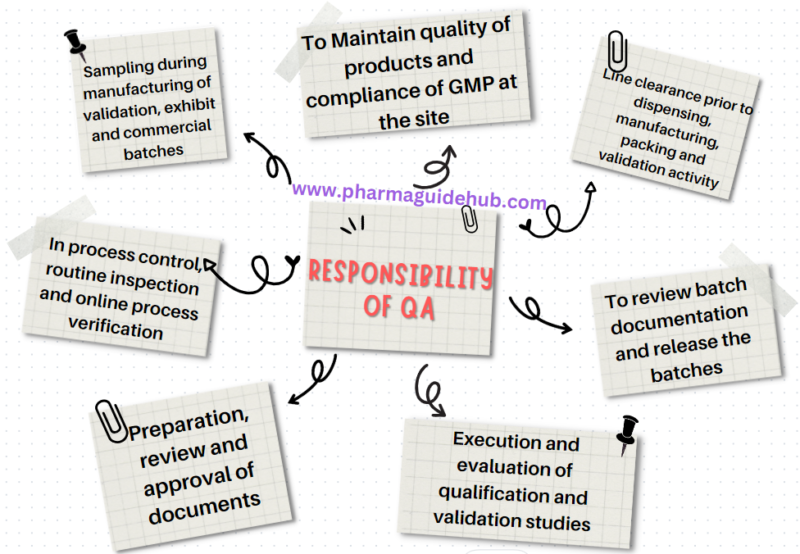
- Responsibility of Quality Assurance:
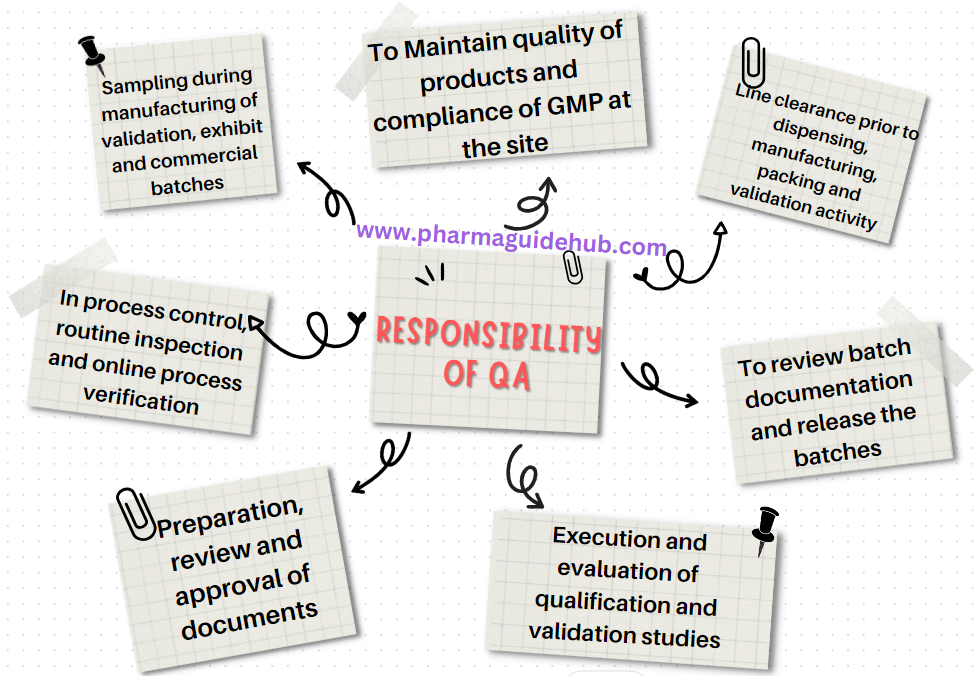
- Responsible for overall quality of products and compliance of GMP at the site.
- Line clearance prior to dispensing, manufacturing, packing and validation activity.
- Sampling during manufacturing of validation, exhibit and commercial batches.
- In process control, routine inspection and online process verification and documentation.
- Preparation, review and approval of documents such as SOP, BMR, BPR, SMF, VMP, APQR, MFR validation protocols/report and qualification protocols/report.
- Execution and evaluation of qualification and validation studies.
- To evaluate and authorize rework or reprocessing of the batch.
- To review batch documentation and release the batches for distribution.
- To involve in quality control functions to ensure GLP and data authenticity.
- Review and approval of work sheet, COA, raw and packing material specification, semi- finished specification, finished product specification and stability protocol.
- To review stability data generated at site and submit to RA for dossier preparation.
- To control, distribute, retrieve and destruction of the master and executed documents such as BMR, BPR, analytical work books, master SOPs, protocols, reports, log books.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/responsibility-of-qa-in-pharmaceutical/
- Verification of calibration, cleaning of instruments and manufacturing equipments.
- Participation in technology transfer process from F & D and other manufacturing site.
- To ensure implementation of quality manual/quality policy, guidance documents and directives.
- To monitor changes in the official monographs and ensuring its implementation at sites.
- To conduct the internal audit, lead the site for external audits and their compliance.
- To ensure personnel training and its evaluation at site.
- Vendor qualification.
- Approval of technical agreements.
- To ensure issuance, control and retrieval of art work and shade card.
- Responsible for risk assessment studies.
- Responsible for investigation and closing of OOS, OOT, deviation, incident, change control and market complaints.
- Responsible for preparation & review the license application to FDA for additional products, COPP, FSC and other FDA related documents before submission.
- To ensure the accuracy, integrity, legibility and availability of documents. Instruction documents should be free from errors and available in writing.
- Any activity covered by the GMP guide that is outsourced should be appropriately defined, agreed and controlled in order to avoid any misunderstanding which could result in a product or operation of unsatisfactory quality.
- QA Head/Designee:
- Responsible for overall quality of products and compliance of GMP at the site.
- To coordinate with Production, HR, Engineering, QC and Warehouse departments.
- Shall be the approving authority for all the master documents such as BMR, BPR SOPs, qualification documents of analytical and manufacturing equipments, specifications, COA, validation protocols, Market complaints, deviation/Incidents, change control, CAPA, OOS and OOT results.
- Release the batches for distribution.
- Coordination with cross functional team, RA, F & D for Review & approval of analytical technology transfer and method validation documents.
- To evaluate and authorize rework or reprocessing of the batch.
- Responsible for approval of technical agreements.
- Responsible for approval of Site Master File,VMP,QM, SOPs, SM, TM & other documents.
- Responsible for approval & investigation of the market complaint and prepare response and forward it for further review.
- To approve the CAPA and comment on the same. Ensure implementation of CAPA.
- To decide, monitor & ensure the batch recall.
- Coordination with Regulatory Affairs for preparation and filling of dossiers.
- To liaison with local FDA for inclusion/grant of license and certifications.
- To lead the site for internal, external GMP audits and their compliance.
- To approve the qualification, validation and revalidation activities and their compliance at the site as per validation master plan.
- To ensure personnel training and its evaluation at site.
- To ensure the shop floor activities and ensure day to day compliance.
- To interact with F & D, Purchase, RA for artwork, vendor qualification and other non conformance issues related to products manufactured at the site.
- To coordinate with HR for recruitment of QA personnel and their induction training.
- To control the budget expenditure.
- Approval of self-audit action plan.
- Approval of destruction/disposal.
- Collecting information and providing Guidelines for personnel knowledge update through information sharing and /or trainings. Advising on training requirements, conducting training on Quality Manual /Quality Policy and monitoring the training activity in the company. Support preparation of training modules and circulating the same to sites.
- Suggesting improvements in product presentation/ packing /quality.
- To monitor changes in the official monographs, guidelines and ensuring its implementation at sites.
- Evaluation & approval of risk assessment in the site.
- To ensure the compliance with validation master plan and requalification of utility, equipments, procedures and systems as per the frequency described.
- To ensure the protocols, related qualification, validation documents and their summary report and related formats of engineering, manufacturing and testing instruments.
- To conduct vendor audits.
- Appraise customer’s requirements and make sure they are satisfied.
- QA Asst. Manager/Designee:
- Preparation of Site Master File, VMP, QM, SM, TM & other documents.
- Monitoring and review of annual product quality review.
- To involve in quality control functions and ensure GLP and data authenticity.
- To ensure issuance, control and retrieval of Art work and shade card.
- To review the executed worksheets / specifications of raw material, packing material, semi-finished and finished product.
- To review the batch documents and QC data and give clearance for release for finished products.
- Review of stability protocols and stability data with coordination of QC and inform to QA head/Designee about the outcome of the stability data and submit to RA for dossier submission purpose.
- Review of calibration records of analytical instruments.
- To monitor changes in the official monographs and inform to QA Head/Designee.
- To review the SOPs and its implementation.
- To ensure the in process testing and compliance during operations.
- To evaluate and approve extra material/ excess return request made by production.
- To assist in investigation of the deviations/ incident, non conformances, market complaint, OOS and OOT.
- To support preparations at site for external audits. Help in preparation of compliance reports.
- To review the change control proposed and to ensure closure of the change controls, post implementation after conducting impact assessment.
- To prepare monthly report and furnished required information to QA Head /Designee.
- To coordinate and conduct internal audits of GMP compliance.
- Allocate the responsibility to the IPQA team for the shift operations if applicable.
- To conduct training for IPQA personnel.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/responsibility-of-qa-in-pharmaceutical/
- To prepare answers to the queries received from Regulatory agencies on the submissions and RA send the draft for review to QA. On confirmation prepare the final version and send it to Regulatory Affairs department, for onward submission to respective Regulatory agencies.
- To prepare & review the license application to FDA for additional products, COPP, FSC and other FDA related documents before submission.
- In the absence of QA head/Designee, the responsibility shall be delegated upward to QA Asst. Manager/Designee.
- QA Executive/Designee:
- To develop efficient filling system of all QA documents by assigning file numbering system, prepare master index, lists of documents and ensure correct filing of documents.
- To maintain, destruction records and ensure safe destruction of obsolete records.
- Preparation of SOP’s.
- Preparation & review of master formulae Record.
- Preparation & Review of batch manufacturing record and batch packing record.
- Review of scale up (Optimization) activity reports.
- Assist in execution of validation and qualification activities.
- Customer complaint investigation.
- Review of filled BMR/BPR.
- Logging of CAPA, MC, OOT, OOS, Deviation/Incident, Change control & other documents.
- Review & compliance of cleanliness and environmental conditions in manufacturing area prior to and during manufacturing.
- Monitoring of destruction/disposal and reprocessing activities; if any.
- To Prepare & review of Annual Product Quality Review.
- To monitor IPQA activities on shop floor randomly.
- To coordinate for annual training of personnel in the Site.
- Ensure the maintenance of control sample and stability sample.
- Responsible for issuance, retrieval, retention and destruction of documents such as Batch Manufacturing Record and Batch Packaging Record, SOP, formats, logbooks, analytical worksheets, specification, Standard testing procedure, General testing procedure, material bin card.
- QA Officer/ Sr. Officer:
- To check of cleanliness and environmental conditions in manufacturing area prior to and during manufacturing.
- To maintain the inward/outward records in the documentation cell.
- Collection of data for annual product quality review.
- To carryout Line clearance checks.
- To carryout In-process checks in Warehouse, manufacturing & Packing departments.
- To check on line documentation.
- To check SOP compliance on shop floor.
- Sampling of semi-finished and finished product.
- Swab and rinse water sampling.
- Control sample & Stability sample collection, storage and monitoring.
- Checking of instrument log books, calibration record & other document in production, QC, Engineering & Warehouse.
- REFERENCES:
Not Applicable
- ANNEXURES:
Not Applicable
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
| Control copy No. 1 | : | Head Quality Assurance |
| Control copy No. 2 | : | Head Production |
| Master Copy | : | Quality Assurance Department |
- ABBREVIATIONS:
| SOP | : | Standard operating procedure |
| QA | : | Quality assurance |
| QC | : | Quality control |
| HR | : | Human resource |
| IPQA | : | In-process quality assurance |
| CAPA | : | Corrective action & Preventive action |
| BMR | : | Batch Manufacturing Record |
| BPR | : | Batch Packaging Record |
| OOS | : | Out of Specification |
| OOT | : | Out of trend |
| RA | : | Regulatory affairs |
| GMP | : | Good manufacturing practices |
| FDA | : | Food Drug & Administration |
| GLP | : | Good Laboratory Practice |
| F&D | : | Formulations & development |
| FAT | : | Factory acceptance test |
| COA | : | Certificate of analysis |
| COPP | : | Certificate of Pharmaceuticals products |
| FSC | : | Free sale certificate |
| SMF | : | Site Master File |
| VMP | : | Validation Master Plan |
| QM | : | Quality Manual |
| TM | : | Training Manual |
| SM | : | Safety Manual |
| MC | : | Market Complaint |
- REVISION HISTORY
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/responsibility-of-qa-in-pharmaceutical/
Frequently Asked Question?
Q1: What is the primary role of Quality Assurance in the pharmaceutical industry? A1: The primary role of Quality Assurance in the pharmaceutical industry is to ensure that products are manufactured, tested, and released in compliance with regulatory standards, industry best practices, and company policies. QA is responsible for maintaining and improving the quality management system to guarantee the safety, efficacy, and quality of pharmaceutical products.
Q2: What are the key responsibilities of a QA professional in the pharmaceutical industry? A2: QA professionals are responsible for developing and implementing quality systems, conducting audits, reviewing and approving documents, ensuring compliance with regulatory requirements, investigating and resolving quality issues, and facilitating continuous improvement initiatives. They play a crucial role in maintaining quality throughout the product lifecycle.
Q3: How does QA contribute to regulatory compliance in the pharmaceutical industry? A3: QA is responsible for establishing and maintaining systems that ensure compliance with various regulatory requirements, including Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and Good Clinical Practices (GCP). QA conducts audits, reviews documentation, and oversees processes to ensure adherence to regulatory standards.
Q4: What is the role of QA in documentation control? A4: QA is responsible for establishing and maintaining a robust documentation control system. This includes reviewing and approving documents such as Standard Operating Procedures (SOPs), batch records, and validation protocols. QA ensures that documentation is accurate, up-to-date, and in compliance with regulatory requirements.
Q5: How does QA handle deviations and non-conformances in the pharmaceutical manufacturing process? A5: QA is responsible for investigating deviations and non-conformances to determine their root causes. They work with other departments to implement corrective and preventive actions (CAPA) to address the issues identified. The goal is to prevent recurrence and improve overall processes.
Q6: How does QA ensure the quality of raw materials and finished products in the pharmaceutical industry? A6: QA establishes and implements procedures for the qualification and monitoring of suppliers, including the evaluation of raw material suppliers. They also conduct inspections and reviews to ensure that raw materials and finished products meet established quality standards before being used or released to the market.
Q7: How does QA contribute to continuous improvement in the pharmaceutical industry? A7: QA plays a vital role in continuous improvement by monitoring processes, analyzing data, and identifying areas for enhancement. This involves implementing CAPA measures, conducting quality risk assessments, and promoting a culture of continuous improvement throughout the organization.
Q8: What are the challenges faced by QA professionals in the pharmaceutical industry? A8: Challenges may include staying updated with evolving regulatory requirements, managing complex supply chains, handling diverse product portfolios, and ensuring a balance between compliance and operational efficiency. QA professionals also need to navigate the integration of new technologies and innovations in the industry.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/responsibility-of-qa-in-pharmaceutical/