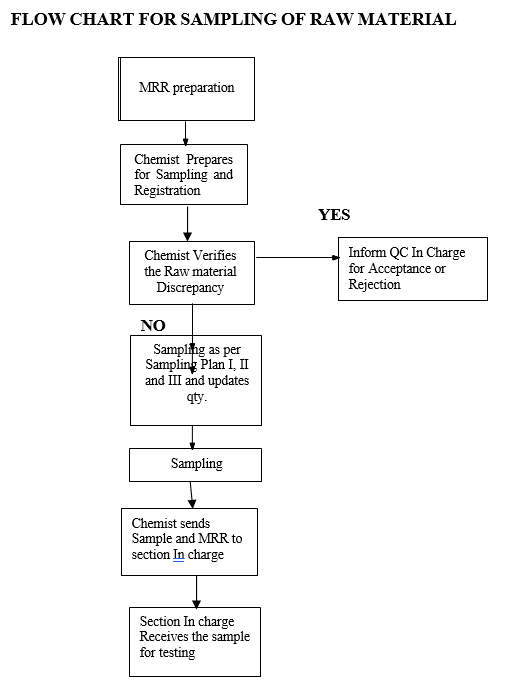
- PROCEDURE:
- On receipt and acceptance of the Raw material, the stores personnel shall quarantine the material and prepare the MRR by forwarding the vendor COA to Quality control Department stating the MRR No & Lot No at the bottom of the COA.
- The Technical Assistant Stores shall arrange the under-test materials for sampling.
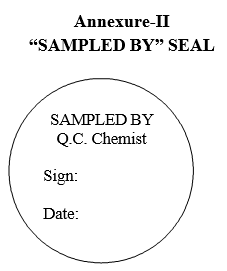
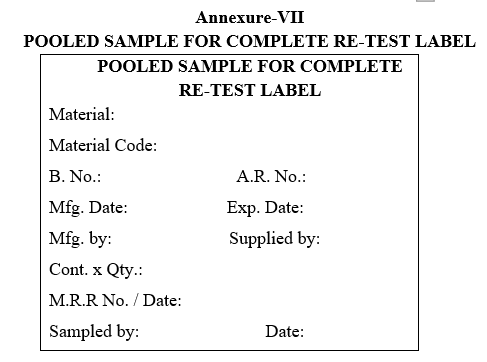
- As per MRR the sampler shall prepare the labels such as “SAMPLE FOR ANALYSIS”, “POOLED SAMPLE FOR COMPLETE ANALYSIS” and “POOLED SAMPLE FOR COMPLETE RE-TEST” with complete details given in MRR and the sampler should be ready with “SAMPLED BY SEAL” to seal on UNDER TEST LABEL.
- The sample shall be registered and assign the AR No as per the SOP.
- Sampler shall follow the gowning procedure as per SOP.Operate and clean the Sampling Isolator as per the SOP.
- Operate the analytical balance as per SOP and verify calibration status of the balance before to its use as per the SOP.
- The material shall be transferred to the sampling area.
- The sampler shall collect appropriate sample quantity as required for respective test parameter as per the respective STP.
- The total sampled quantity should be 3X to the quantity required as per the STP (1X-Reference sample, 2&3X- is Analysis and Re-Test samples).
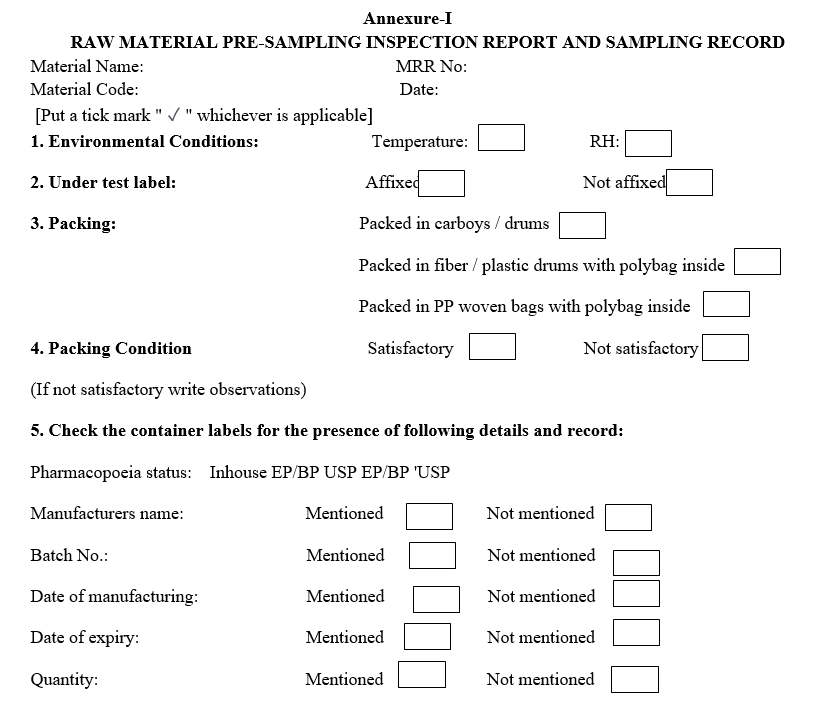
- The sampler shall check the material as per the “Raw Material Pre Sampling Inspection Report and Sampling Record”.
- If the sampler finds any discrepancy in the material the same shall be informed to the In charge –Raw Material Release/In charge Ware House for necessary actions.
- In charge Raw Material Release shall take the necessary corrective action based on the observation and shall decide to accept or reject in consultation with the In charge Quality Control.
- Sampler shall sample with cleaned and suitable sampling tool as mentioned below based on the type and size of the container.
- Transfer the materials to be sampled to the safe working zone in a sequence; in such a way that first container is sampled first and so on.
- Ensure that only one container is taken up for sampling and sample one batch at any time.
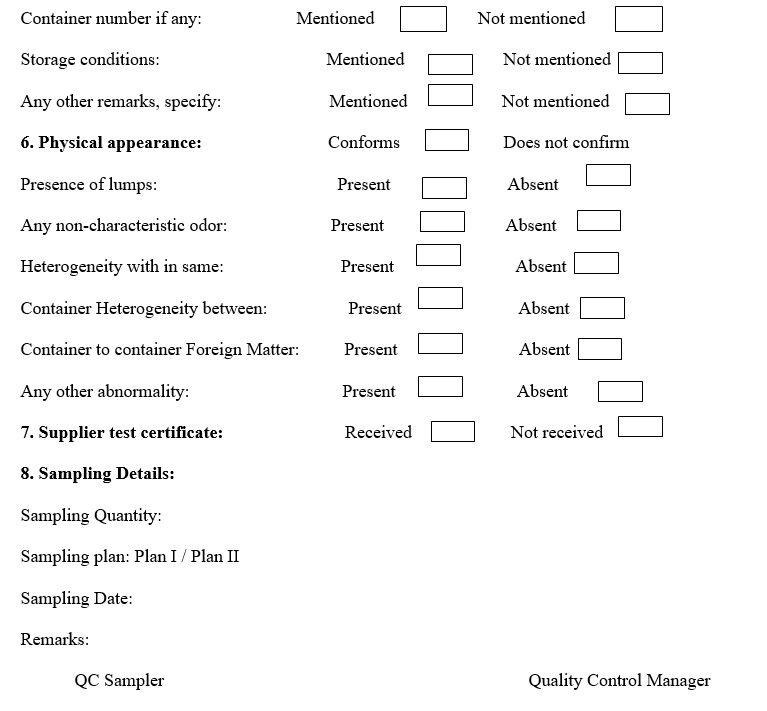
- Before opening the packs / containers for sampling, the packs are visually examined for any damages and observations are recorded in the Annexure-I.
- Break the seals outside of the sampling room.
- Physically damaged containers/bags, where innermost packing has been damaged, shall not be considered for sampling and the same shall be rejected.
- In case of any discrepancies, inform to In charge-Raw material release /Warehouse In charge For necessary action.
- Sample the material using suitable sampling tools and sampling plan described below Sampler shall use containers or poly bags for collecting the samples.
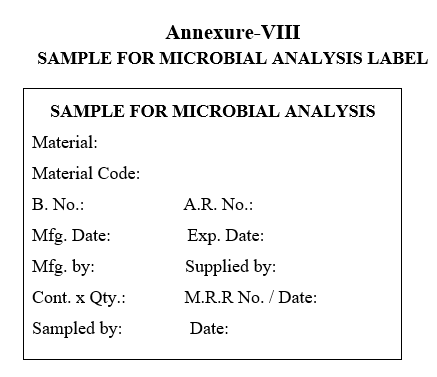
- The samples shall be collected first for Microbiological testing, wherever applicable, in sterile glass bottles/poly bags and label as “SAMPLE FOR MICROBIAL ANALYSIS” as per the Annexure-VIII.
- Sample quantity for Microbiology is about 20 grams or as specified.
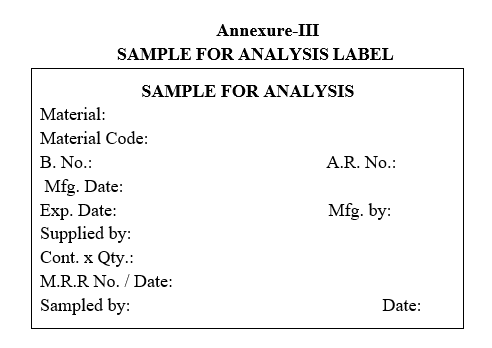
- As per the sampling plan, collect the samples from those many containers to perform Identification tests and label as Sample for analysis.
- Collect the sample from the individual containers as per the sampling plan for making a composite sample, for a complete analysis and Reference sample.
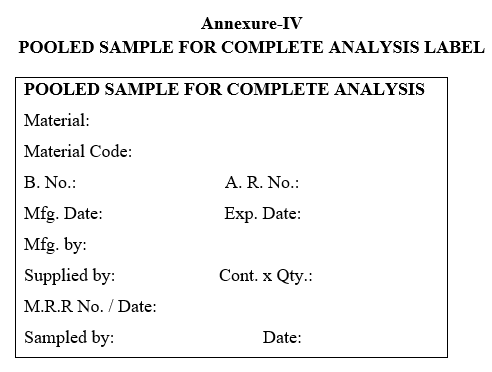
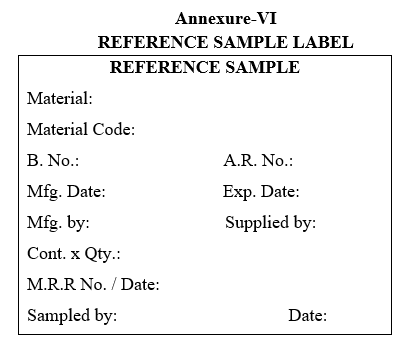
- Label the containers as per the “POOLED SAMPLE FOR COMPLETE ANALYSIS” and “REFERENCE SAMPLE” as per the Annexure-VII respectively.
- Example: Total containers received: 10 No’s
- Sample for analysis: 10 No’s
- Pooled sample for analysis: 1 No (1 to 10)
- After sampling reseal the internal pack with cable tie, ensuring that the sealing is proper and complete the external pack with tamper proof seals.
- Avoid tapping the scoop to remove material as to prevent the segregation of the sample.
- Do not put back the sampled material into the original container/bag.
- The sampling details and observations shall be recorded in the raw material sampling and visual inspection report.
- Put a seal on the UNDER TEST LABEL present on the sampled container and transfer the containers back to the respective under test quarantine areas.
- The sampler shall update the sampling quantity.
- Sampler shall send the sample, MRR and suppliers COA along with the “Raw Material Pre Sampling Inspection Report and Sampling Record” to the concern in -charge of Raw Material testing and release.
- Clean the Sampling Isolator as per the SOP.Transfer the sampling tools to the washing area and clean as per the SOP
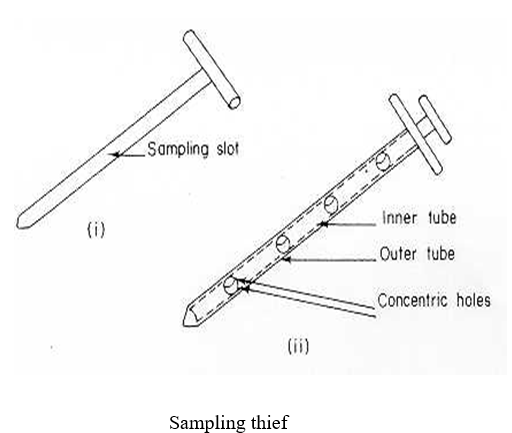
- Sampling Tools
- Sampling tools shall be used based on the Size / Volume of the container and the occupancy of the material in the supplied container.
- Typical Example of a Scoop and Sampling Thieve are shown below.
| TYPE | Pack | Pack Size | Material Occupancy | Sampling Tool |
| Solid | Container | 50 kg | 25% | SS Thieve Sampler (Length : 1 feet) |
| 50 kg | 50% | SS Thieve Sampler (Length : 2 feet) | ||
| 50 kg | 75% | SS Thieve Sampler (Length : 3 feet) |
- The sampling tool shall be selected in such a way that the sample shall be collected from the top, middle and bottom.
- Wherever the use of thieve sampler is not feasible, SS Scoops/Spatulas shall be used as per the above described procedure.
- Sampling Plan:Plan I (For Actives)
- Sampling shall be done from all the containers for active ingredient for Identification test.
- One identification shall be carried out preferably by IR Method / HPLC/ chemical identification method wherever applicable OR as per the method given in the Standard Testing Procedure.
- The “POOLED SAMPLE FOR COMPLETE ANALYSIS” collected from all the containers shall be used for complete analysis.
- Each sampled container shall be carried out for identification and the pooled sample shall be done for complete analysis.
Plan II (For in Actives):
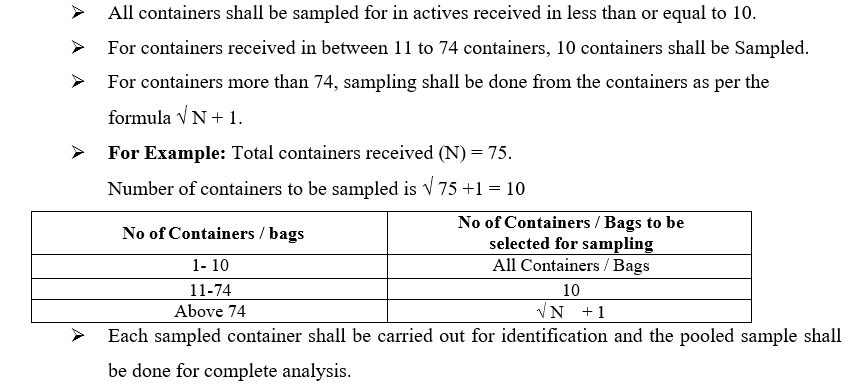
- Operation of the Sampling Tools: Operation of the Sampling Thieve:
- The procedure shall be applicable to sample Solid Raw Materials received in HDPE / Fiber Drums Using a Thieve sampler.
- Smoothly insert the sampling thief to reach the bottom of the container/Drum.
- Open the thief by turning the clockwise direction to collect the sample in three slots representing the top, middle and bottom layers of the containers/drums and close the thief by turning anti clockwise direction.
- Take out the sampling thief from the container/drum, open the thief by turning clockwise direction and collect the material with the help of spatula into the sampling container.
- Repeat above steps to collect sample from each container.
- During sampling, once material drawn from the container shall not be returned to the original container.
- Procedure for Re-test:
- The stores personnel shall quarantine the material and prepare the retest request and send to Quality control Department.
- On receipt of retest request, quality control personnel shall check the status of material for Under Test status.
- The Stores personal shall arrange the under test materials for sampling.
- The sampler shall view and prepare the “POOLED SAMPLE FOR COMPLETE ANALYSIS” and “POOLED SAMPLE FOR COMPLETE RE-TEST” labels with complete details given in retest request and the sampler should be ready with SAMPLED BY SEAL to seal on UNDER TEST LABEL.
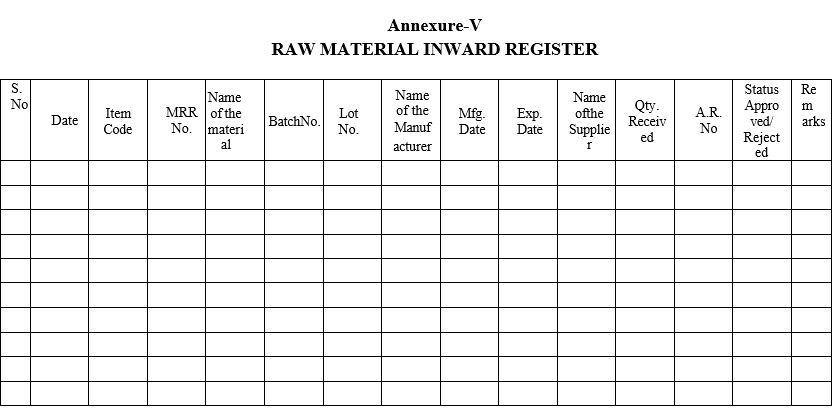
- The sample shall be registered as per Annexure-V and assign the AR No. as per SOP.
- Sampler shall follow the gowning procedure as per SOP.
- Operate the Sampling Isolator as per the SOP (If required).
- Operate the analytical balance as per SOP and verify calibration status of the balance before use as per the SOP.
- The material shall be transferred to the sampling area.
- The sampler shall collect appropriate sample quantity as required for respective test parameter as per the respective STP.
- The total sampled quantity should be 2X to the quantity required as per the STP (1&2X- is Analysis and Re-Test samples).
- The sampler shall check the material as per the “Raw Material Pre Sampling Inspection Report and Sampling Record”.
- If the sampler finds any discrepancy in the material the same shall be informed to the in charge –Raw Material Release/In charge Warehouse for necessary actions.
- In charge of Raw Material Release shall take the necessary corrective action based on the observation and shall decide to accept or reject in consultation with the in charge Quality Control.
- Sampler shall sample with cleaned and suitable sampling tool as mentioned below based on the type and size of the container.
- Transfer the materials to be sampled to the safe working zone in a sequence; in such a way that first container is sampled first and so on.
- Ensure that only one container is taken up for sampling and sample one batch at any time.
- Before opening the packs/containers for sampling, the packs are visually examined for any damages and observations are recorded in Annexure-I.
- Break the seals outside of the sampling room.
- Physically damaged containers/bags, where innermost packing has been damaged, shall not be considered for sampling and the same shall be rejected.
- In case of any discrepancies, inform to in charge-Raw material release/Warehouse In charge for necessary action.
- Sample the material using suitable sampling tools and sampling plan described below.
- Samplers shall use containers or poly bags for collecting the samples.
- If the raw material is light sensitive, after sampling wrap the container with a black polythene bag or aluminum foil and seal.
- The samples shall be collected first for Microbiological testing, wherever applicable, in sterile glass bottles/poly bags and label as “SAMPLE FOR MICROBIAL ANALYSIS”.
- Sample quantity for Microbiology is about 20 grams or as specified.
- Collect the sample from the individual containers as per the sampling plan for making a composite sample, for a complete analysis.
- Label the containers as per the “POOLED SAMPLE FOR COMPLETE ANALYSIS” respectively.
- Example: Total containers received: 10 No’s
- Containers sampled: 10 No’s
- Pooled sample for analysis: 1 No (1 to 10)
- After sampling reseal the internal pack with cable tie, ensuring that the sealing is proper and complete the external pack with tamper proof seals.
- Avoid tapping the scoop to remove material as to prevent the segregation of the sample.
- Do not put back the sampled material into the original container/bag
- The sampling details and observations shall be recorded in the raw material sampling and visual inspection report.
- Put a seal on the UNDER TEST LABEL present on the sampled container and transfer the containers back to the respective under test quarantine areas.
- The sampler shall update the sampling quantity.
- The sampler shall send the sample, retest request and suppliers COA along with the “Raw Material Pre Sampling Inspection Report and Sampling Record” to the concern in -charge of Raw Material testing and release.
- Clean the sampling booth and record the details as per SOP
- Transfer the sampling tools to the washing area and clean as per the SOP.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Raw Material Pre-Sampling Inspection Report and Sampling Record |
| Annexure-II | “Sampled by” seal |
| Annexure-II | Sample For analysis Label |
| Annexure-IV | Pooled Sample for Complete analysis Label |
| Annexure-V | Raw material inward register |
| Annexure-VI | Reference Sample Label |
| Annexure-VII | Pooled sample for complete Re-Test label |
| Annexure-VIII | Sample For Microbial Analysis Label |
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/sampling-of-raw-material/
FLOW CHART FOR SAMPLING OF RAW MATERIAL
Annexure-I
RAW MATERIAL PRE-SAMPLING INSPECTION REPORT AND SAMPLING RECORD
Annexure-II
“SAMPLED BY” SEAL
Annexure-III
SAMPLE FOR ANALYSIS LABEL
Annexure-IV
POOLED SAMPLE FOR COMPLETE ANALYSIS LABEL
Annexure-V
RAW MATERIAL INWARD REGISTER
Annexure-VI
REFERENCE SAMPLE LABEL
Annexure-VII
POOLED SAMPLE FOR COMPLETE RE-TEST LABEL
Annexure-VIII
SAMPLE FOR MICROBIAL ANALYSIS LABEL
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/sampling-of-raw-material/
