- OBJECTIVE
To lay down a procedure for standard operating procedure on collection and handling of control sample.
- SCOPE:
This SOP is applicable for standard operating procedure on collection and handling of control sample at {Company Name} {Company Location}.
- RESPONSIBILITY:
- Executives/Officer In process Quality Assurance (IPQA) shall be responsible for collection of control sample.
- Executives/Officer Quality Assurance (QA) shall be responsible for storage, issuance, periodic observation, and disposal of control samples.
- Manager QA shall be responsible for checking and compliance of the SOP.
- ACCOUNTABILITY:
QA Head shall be accountable for the implementation of SOP.
- PROCEDURE:
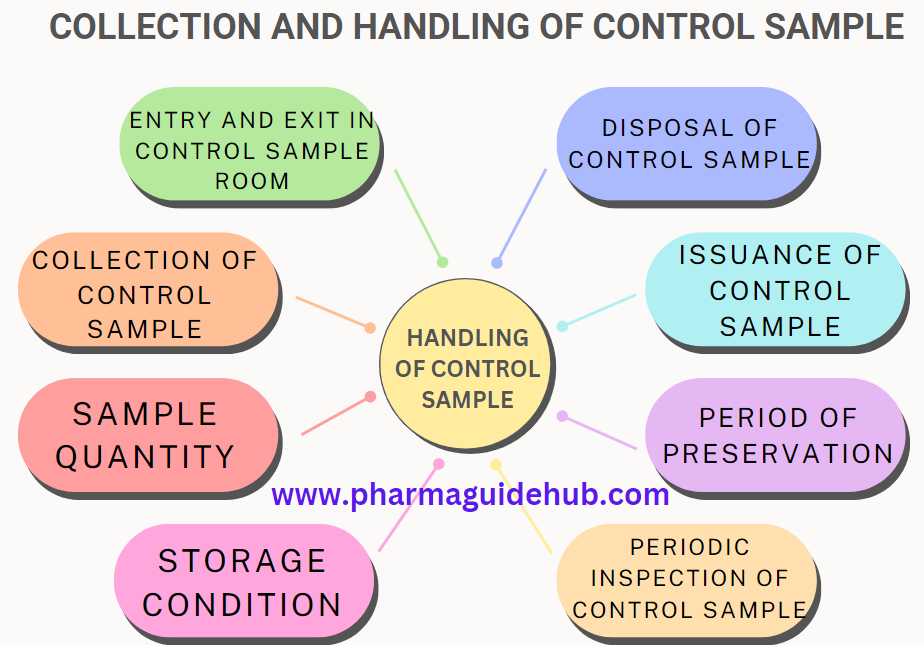
A control sample refers to a specific amount of material – it can be a starting material, packaging material, drug substance, or even the final drug product itself – collected from a particular batch. This sample is then stored under controlled conditions throughout the shelf life of the batch.
The terminology ‘control sample’ referred in this SOP shall be applicable for the equivalent terms such as Reserve sample/ Reference sample/ Retention sample referred in different texts or guidelines or regulations.
- Entry and Exit in Control Sample Room
Control Sample room shall be under access control. Entry in the control sample room shall be restricted, only the person having access is allowed to enter in the control sample room. Visitors or auditors are allowed in the control sample room only with the authorized person.
- Collection of Control Sample
- Control samples shall be collected randomly for each batch of all finished products during packing.
- Control samples shall be collected by IPQA Officer/Executive in such a way that it represents complete batch packing activity (withdraw sample from start, middle and end of packing) and record the details in Annexure-I.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/standard-operating-procedure-on-collection-and-handling-of-control-sample/
- Sample Quantity
- Before collecting control sample ensure correctness of batch number, Mfg. Date, Exp. Date, Mfg. License No., MRP & any other instructions as specified in the batch record.
- The quantity of control samples to be collected shall be a minimum twice of the quantity required for complete analysis of the finished product and it should be collected in such a way so as not to disturb the primary packing configuration.
- 200 Tablets shall be withdrawn as control sample for product having Standard wt. 250 mg and above; in case product having Standard wt. up to 250 mg, 300 tablets shall be withdrawn as control sample.
- In case of bulk packing 10 filled bottles shall be collected as a control sample.
- If a batch is split in two or more child batches, control sample shall be collected from each child batch and entry shall be made in respective BPR.
- In case of Physician sample (PS) packing 2 Units shall be collected for the control sample.
- Storage Condition
- Control sample shall be kept in control sample room after stamping the ‘CONTROL SAMPLE’ on every carton of the product.
- Control sample shall be segregated mfg. date and exp. date wise and placed in corrugated box / container. A label shall be pasted on corrugated box / container having Product Name, Batch no., Mfg. date and Exp. date.
- Control samples shall be stored at room temperature and RH is not applicable for domestic products. RH shall be monitored based on regulatory requirement for the products.
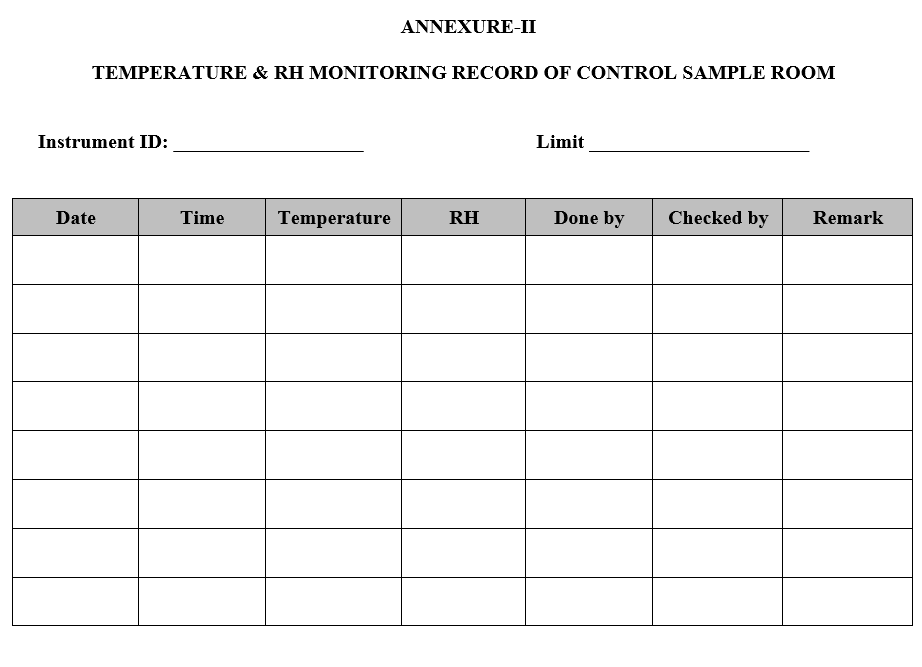
- The temperature and relative humidity of control samples storage area shall be monitored and recorded regularly twice in a day (preferably morning & evening all the working days) in the temperature and relative humidity record as per Annexure-II.
- Periodic Inspection of Control Sample
- The Control samples shall be examined visually by QA executive/Designee once in year up to the expiry of product, for evidence of any physical changes / defects / deterioration in QA module.
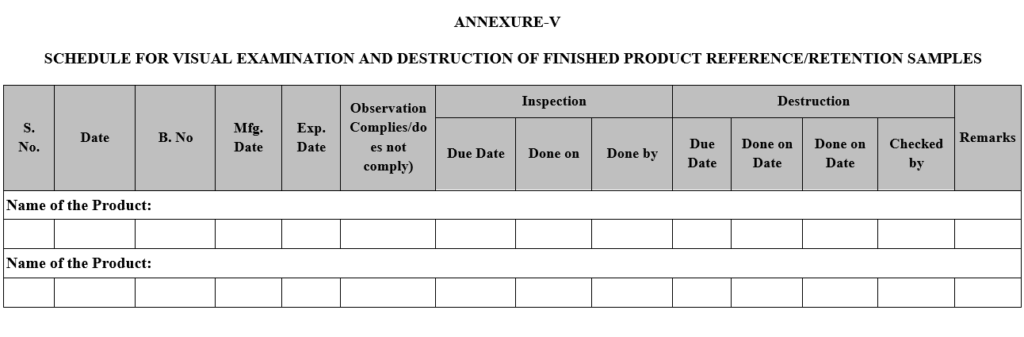
- Schedule for periodic visual inspection of control sample shall be maintained in Visual Inspection and Destruction” as per Annexure V.
- If there is any abnormality observed during inspection, it shall be recorded, and investigation shall be performed as per respective SOP.
- Period of Preservation
The control samples shall be stored for a period of one year after the expiry of the product.
- Issuance of Control Sample
- Control samples shall be issued only in case of
- Market complaint (for investigation & analysis)
- Registration/ reference samples are required.
- Submission to regulatory authorities or for any other reference purpose.
- As per party requirement.
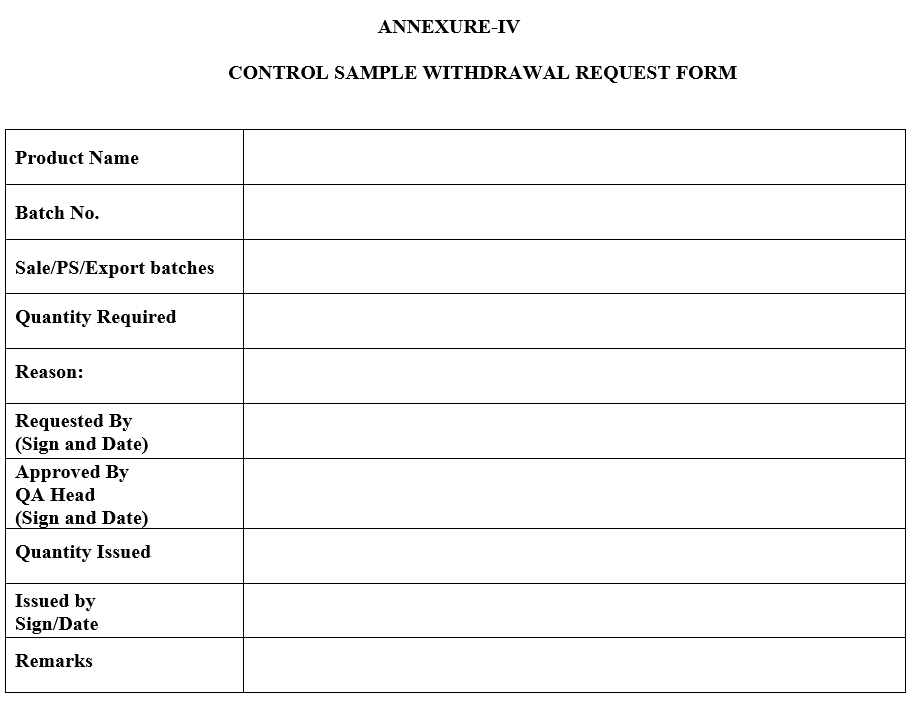
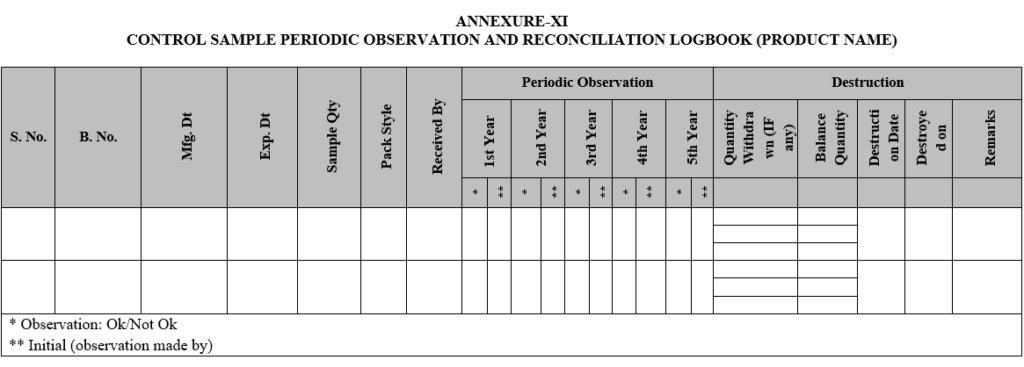
- If control sample required for testing or reference purpose, concern person shall be fill the control sample “Withdrawal Request Form” as per Annexure-VIII and maintain reconciliation record in ‘Control Sample Periodic Observation and Reconciliation Log Book’ as per Annexure-IX.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/standard-operating-procedure-on-collection-and-handling-of-control-sample/
- Disposal of Control Sample
- Control sample of finished products shall be disposed off after the retention period i.e. after one year of expiry date of product.
- QA Officer/Executive shall prepare a list of control samples to be destroyed in advance by referring list take approval from QA manager.
- Withdraw the samples from control sample room & collect it in a corrugated box / container, duly labeled as “EXPIRED CONTROL SAMPLES FOR DESTRUCTION”.
- After getting QA Manager approval control sample shall be destroyed as per respective SOP.
- REFERENCES:
Schedule– M.
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
| Annexure-I | Control Sample Logbook |
| Annexure-II | Temperature and Relative Humidity Record |
| Annexure-III | Control Sample Inward, Inspection and Destruction Register of Finished Product |
| Annexure-IV | Control Sample Withdrawal Request Form |
| Annexure-V | Schedule for Visual Inspection and Destruction |
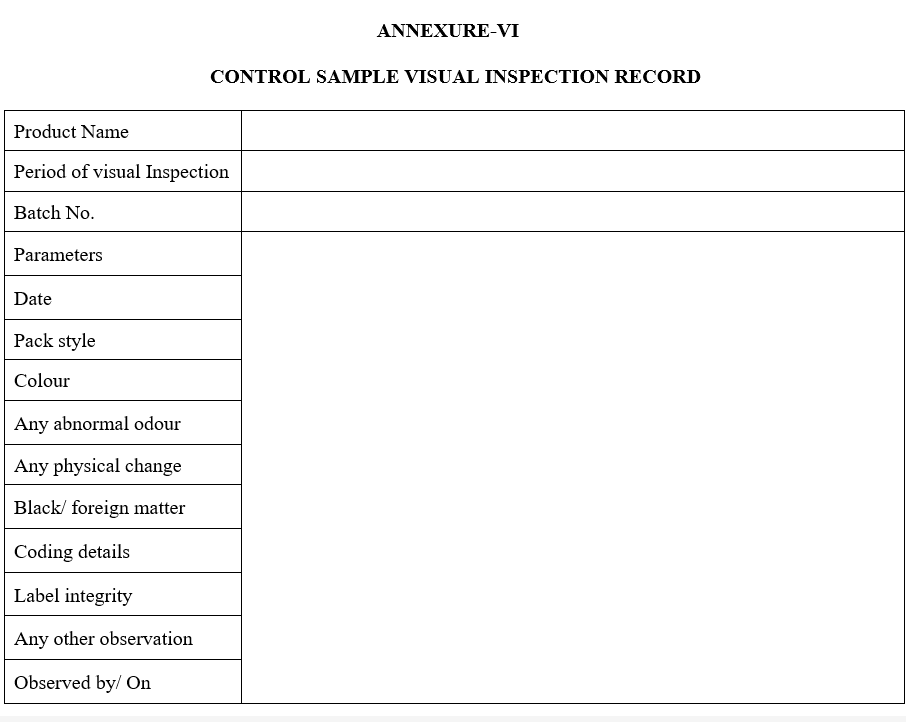
| Annexure-VI | Control Sample Visual Inspection Record |
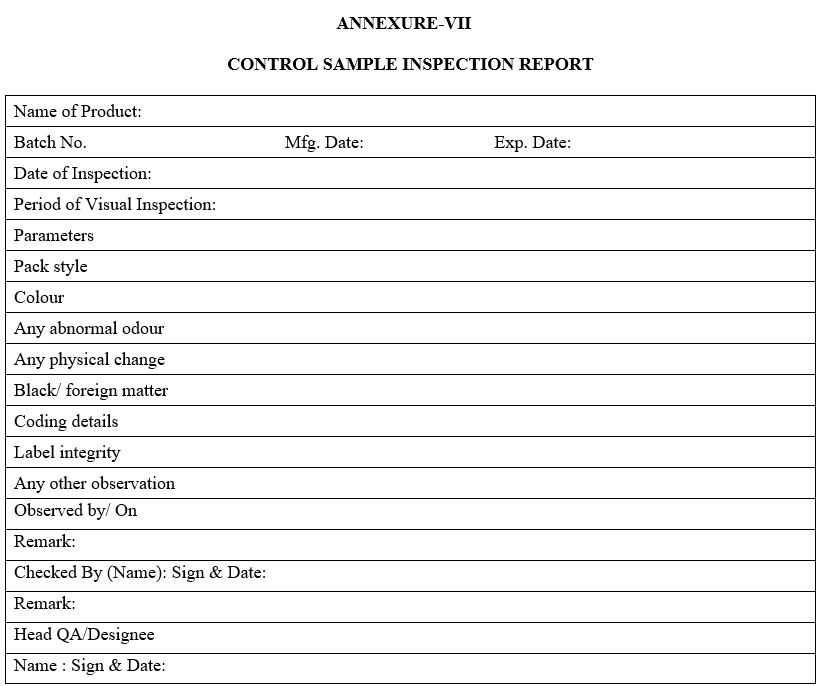
| Annexure-VII | Control Sample Inspection Report |
| Annexure-VIII | Sample Withdrawal Request Form |
| Annexure-XI | Control Sample Periodic Observation and Reconciliation Logbook |
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| QA | : | Quality Assurance |
| SOP | : | Standard Operating Procedure |
| BPR QA | : : | Batch packaging record Quality Control |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
ANNEXURE-I
CONTROL SAMPLE LOGBOOK

ANNEXURE-II
TEMPERATURE & RH MONITORING RECORD OF CONTROL SAMPLE ROOM
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/standard-operating-procedure-on-collection-and-handling-of-control-sample/
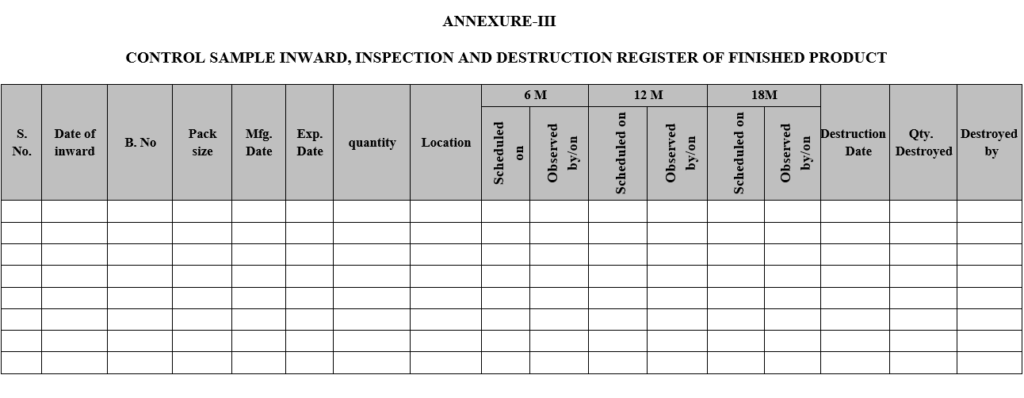
ANNEXURE-III
CONTROL SAMPLE INWARD, INSPECTION AND DESTRUCTION REGISTER OF FINISHED PRODUCT
ANNEXURE-IV
CONTROL SAMPLE WITHDRAWAL REQUEST FORM
ANNEXURE-V
SCHEDULE FOR VISUAL EXAMINATION AND DESTRUCTION OF FINISHED PRODUCT REFERENCE/RETENTION SAMPLES
ANNEXURE-VI
CONTROL SAMPLE VISUAL INSPECTION RECORD
ANNEXURE-VII
CONTROL SAMPLE INSPECTION REPORT
ANNEXURE-VIII
SAMPLE WITHDRAWAL REQUEST FORM

ANNEXURE-XI
CONTROL SAMPLE PERIODIC OBSERVATION AND RECONCILIATION LOGBOOK (PRODUCT NAME)
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/standard-operating-procedure-on-collection-and-handling-of-control-sample/
Frequently Asked Questions?
- Q: What is the purpose of the SOP of control sample management?
- Ans: This SOP outlines the procedures for collecting, storing, inspecting, issuing, and disposing of control samples for finished products.
- Q: What is the definition of a “control sample” in this context?
- Ans: This SOP uses “control sample” interchangeably with terms like “reserve sample,” “reference sample,” and “retention sample” to refer to finished product samples kept for future analysis or investigation.
- Q: How are control samples selected?
- Ans: They are randomly collected from each batch of finished products during packing, including samples from the beginning, middle, and end of the process.
- Q: How much of a control sample is needed?
- Ans: At least twice the amount required for complete product analysis, ensuring enough for any necessary testing.
- Q: Where are control samples stored?
- Ans: In a controlled access room, segregated by manufacturing date and expiry date.
Q: What are the storage conditions for control samples?
Ans: Room temperature for domestic products, with temperature and humidity monitored regularly.
Q: When are control samples issued?
Ans: Only for market complaints, regulatory requirements, reference purposes, or as requested by relevant parties.
Q: How are control sample issuances documented?
Ans: A withdrawal request form and reconciliation log book must be used.
Q:When are control samples discarded?
Ans: One year after the product’s expiry date.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/standard-operating-procedure-on-collection-and-handling-of-control-sample/


