- OBJECTIVE:
- To lay down the procedure for storage and handling of Expired and Rejected Finished Goods.
- SCOPE:
- This SOP is applicable for the procedure for storage and handling of Expired and Rejected Finished Goods at {Company Name} {Location}.
- RESPONSIBILITY:
- WH Executive/Designee – shall be responsible to follow the procedure as per SOP.
- Executive – QA shall be responsible for the physical verification of Finished Goods to be destroyed before transfer to scrap yard.
- Head Warehouse – is responsible for compliance of the SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- Commercial Finished Goods, which were originally dispatched from Finished Goods Stores and over a period of time got expired, shall not be brought back to factory.
- Commercial Finished Goods, which got expired in Finished Goods Stores, shall be stored in ‘Rejected Material Store’.
- Exhibit Finished Goods, which got expired in Finished Goods Stores, shall be stored in the designated area.
- The rejected Finished Goods shall be stored in ‘Rejected Material Store’ under lock and key.
- Restricted access shall be provided for the ‘Rejected Material Store’.
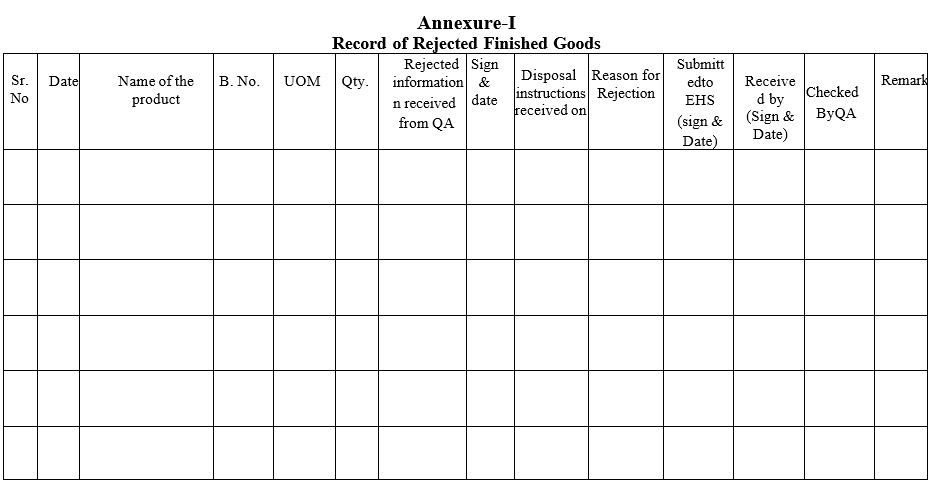
- Details of the Rejected Finished Products shall be entered in the ‘Record of Rejected Finished Goods’.
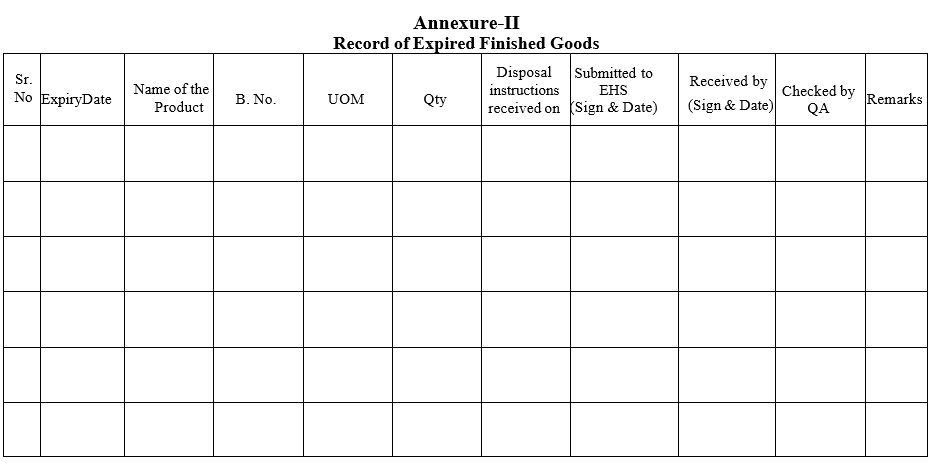
- Details of the Expired Finished Products (commercial) shall be entered in the ‘Record of Expired Finished Goods’.
- For Products pertaining to US market, after submission of Exhibit samples for bio-equivalence /stability/other process monitoring, 10,000 units or less (if the batch size is less than 10,000 units) shall be kept under stated storage condition in F.G Stores up to one year after approval from USFDA and remaining quantity can be destroyed one year after the expiry of the product.
- For Products pertaining to other than US market, after submission of samples for bio-equivalence/ stability/other process monitoring, the remaining quantity shall be kept under stated storage condition in the FG stores up to one year after expiry (or) up to six months after approval from respective health authorities, whichever is earlier.
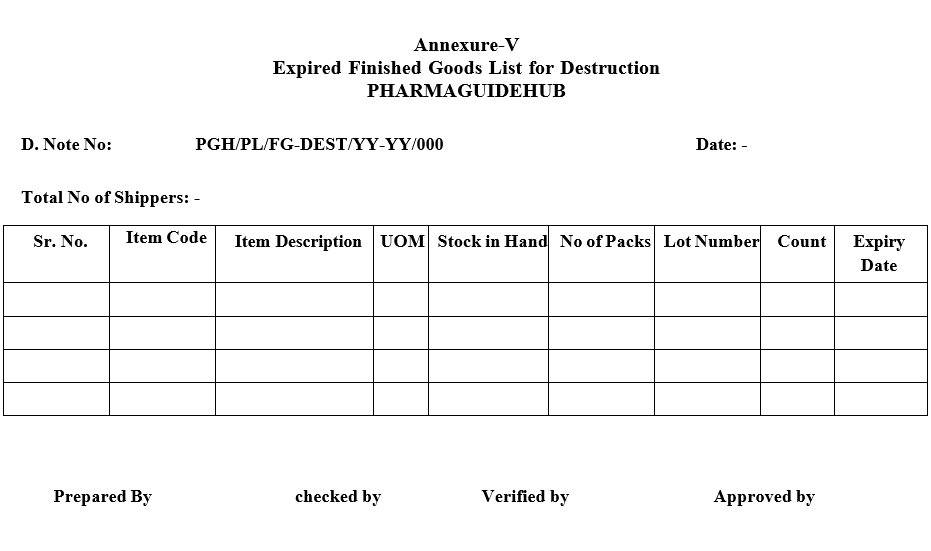
- ‘Expired Finished Goods List for Destruction’ shall be prepared and approvals are obtained for destruction and the approved List of Expired Finished Goods for Destruction shall be forwarded to the Customs Department for obtaining the permission for the destruction.
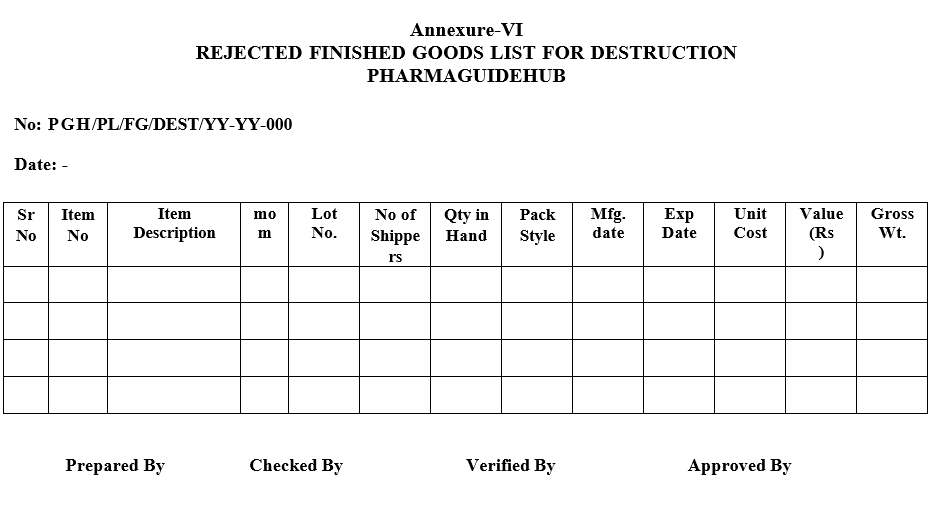
The serial Number of the List of Expired Finished Goods for Destruction shall be generated as ‘PGH/PL/FG-DEST/YY-YY/000’
Where
‘PGH’ denotes Pharmaguidehub
‘/’ denotes (slash sign)
PL denotes Packing List,
‘/’ denotes (slash sign),
‘FG-DEST’ denotes Finished Goods-Destruction,
‘/’ denotes (slash sign),
‘YY-YY’ denotes the Financial Year (For this Financial Year will be denoted as ‘16-17’),
‘/’ denotes (slash sign)
‘000’ denotes the serial number of the List (For example: ‘001,002—–00N’ where ‘N’ is the last serial number
- The ‘Rejected Finished Goods List for Destruction’ shall be generated and approvals shall be obtained for destruction and list shall be forwarded to the Customs Department for obtaining the permission for the destruction.
- After obtaining permission from the Customs department, Expired Finished Goods shall be verified in the presence of QA Personnel as mentioned below and update the stock.
- Warehouse Personnel shall initiate the PNC/MNC as per SOP.
- The copy of the approved list shall be attached to PNC/MNC.After QA verification, Expired Finished Goods shall be transferred to the designated area in the scrap yard for De-Cartooning.
- The Labels of the Plastic Bottles shall be defaced by crossing the labels after de-bottling and same shall be transferred to the scrap yard.
- Tablets/capsules are handed over to EHS for destruction with the documents.
- After de-blistering, Blisters shall be segregated and same shall be transferred to the scrap yard.
- Tablets /capsules are handed over to EHS for destruction with the documents.
- The secondary packing materials such as Cartons, Leaflets and Medication Guides shall be torn into 2 pieces and shall be collected in poly-bags and transferred to scrap yard.
- The Labels of the outer shippers shall be crossed and the shippers shall be transferred to scrap yard.
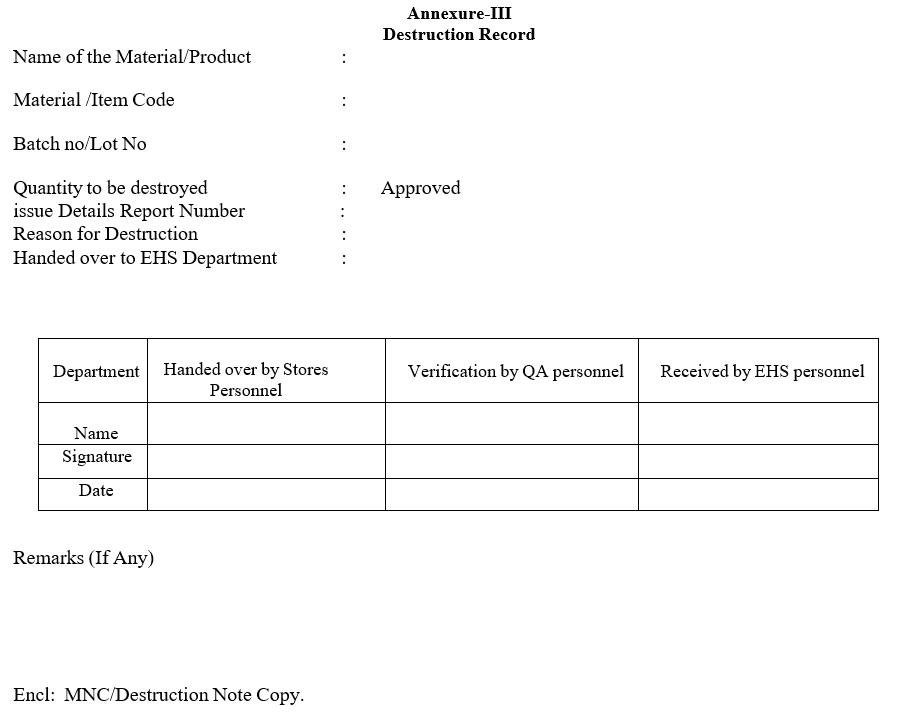
- The duly filled ‘Destruction Record’ and a copy of approved PNC/MNC shall be handed over to EHS Department along with Rejected/Expired Finished Goods for destruction.
- EHS Personnel shall destroy the Rejected/Expired Finished Goods (Tablets/Capsules) as per SOP.
- A copy of the Destruction record shall be obtained by Warehouse Personnel from EHS Personnel after the destruction process.
- Entries shall be completed in the ‘Record of Rejected Finished Goods’.
- List shall be made for the ‘Record of Expired Finished Goods’ after destruction and signed by done by and checked by personnel.
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Record of Rejected Finished Goods |
| Annexure-II | Record of Expired Finished Goods |
| Annexure-III | Destruction Record |
| Annexure-IV | Record of Expired Finished Goods |
| Annexure-V | Expired Finished Goods List for Destruction |
| Annexure-VI | Rejected Finished Goods List for Destruction’ |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Warehouse
- Controlled Copy No. 03 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| PGH | : | Pharmaguidehub |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| API | : | Active Pharmaceutical Ingredients |
| QA | : | Quality Assurance |
| EHS | : | Environment, Health and Safety |
| PNC | : | Process Non-Conformance |
| MNC | : | Material Non-conformance |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
Record of Rejected Finished Goods
Annexure-II
Record of Expired Finished Goods
Annexure-III
Destruction Record
Annexure-IV
Record of Expired Finished Goods
Annexure-V
Expired Finished Goods List for Destruction
Annexure-VI
REJECTED FINISHED GOODS LIST FOR DESTRUCTION