- PROCEDURE FOR OSD DOSAGE FORM:
- Movement of product from one stage to next stage is important in pharmaceutical industry so product can be transfer in safe mode and any kind of mixup and contamination can be avoided.
- Procedure for the movement of material:
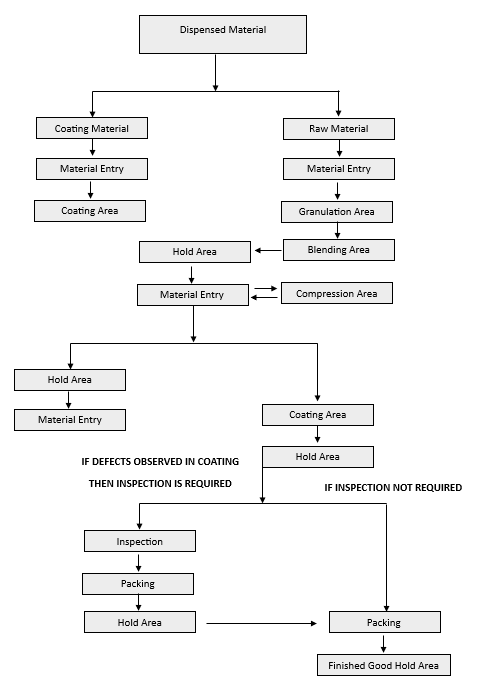
- Unload the in-process material into pre weighed container with double lined poly bags which are already kept in container.
- The materials are unloaded to approximately 80 – 90 % of the holding capacity of the in-process container.
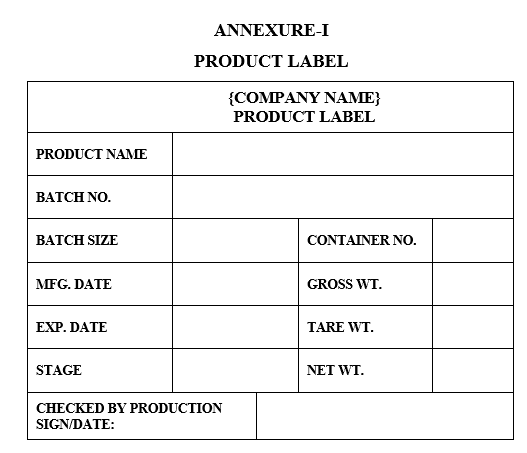
- Affix “PRODUCT LABEL” duly filled as per the Annexure-I to the container.
- Tie the polybags properly and clean the internal and external surface of the container and lid with lint free cloth, then close the container with lid.
- Weigh the Containers in designated area and record the weight in the product label.
- Record the details of weighing in Batch Manufacturing Record.
- Then transfer the material to the Hold Area.
- Place the containers in such a way that the labels are clearly visible.
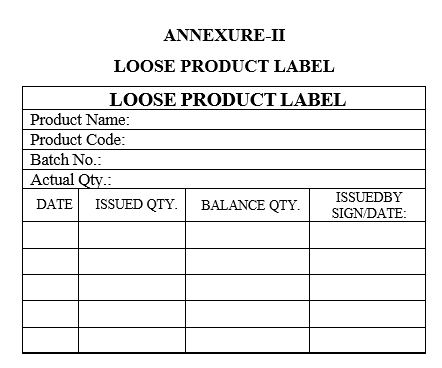
- In case of partial quantity of tablets or capsules are required for the packing activity “LOOSE PRODUCT LABEL” to be affixed to finished product container with duly filled details by concern packing person.
- Each time while taking out partial quantity of tablets or capsules from the container for packing, enter the details in issued quantity and balance quantity of the tablets in “LOOSE PRODUCT LABEL”.
- Maintain the Temperature and Relative Humidity as per the SOP.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Product Label |
| Annexure-II | Loose Product Label |
- ABBREVIATIONS:
| PD | : | Production |
| PPE | : | Personnel protective equipment |
| PAPR | : | Powered air purified respirator |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
ANNEXURE-I
PRODUCT LABEL
ANNEXURE-II
LOOSE PRODUCT LABEL