OBJECTIVE:
To lay down the procedure for the storage and movement of semi-finished and finished product.
SCOPE:
This SOP is applicable to the procedure for the storage and movement of semi-finished and finished products at Company Name} {Company Location}.
RESPONSIBILITY:
- Production Executive/Designee – is responsible to follow the procedure as per SOP.
- Head Production – is responsible for compliance of the SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
PROCEDURE:
Procedure:
Unload the in process material into pre weighed container with double lined poly bags which are already kept in container.
The materials are unloaded to approximately 80 – 90 % of the holding capacity of the in process container.Affix “PRODUCT LABEL” duly filled as per the Annexure-I to the container.
Tie the polybags properly and clean the internal and external surface of the container and lid with lint free cloth, then close the container with lid.
Weigh the Containers in designated area and record the weight in the product label.
Record the details of weighing in Batch Manufacturing Record.
Then transfer the material to the Hold Area. Place the containers in such a way that the labels are clearly visible.
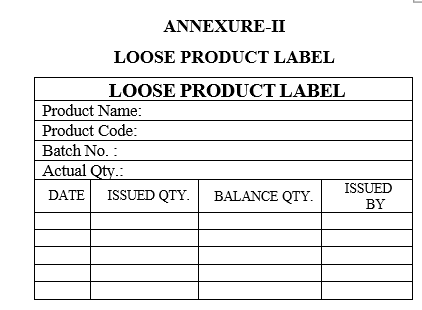
In case of partial quantity of tablets required for the packing activity “LOOSE PRODUCT LABEL” to be affixed to finished product container with duly filled details by concern packing person.
Each time while taking out partial quantity of tablets from the container for packing, enter the details in issued quantity and balance quantity of the tablets in “LOOSE PRODUCT LABEL”.
For starting the compression take Blend from hold area. Ensure approved status and verify the weights as per the BMR in designated area.
Maintain the Temperature and Relative Humidity as per the SOP.
REFERENCES:
Not Applicable
ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Product Label |
| Annexure-II | Loose Product Label |
DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Production
- Master Copy : Quality Assurance Department
ABBREVIATIONS:
| PD | : | Production |
| PPE | : | Personnel protective equipment |
| PAPR | : | Powered air purified respirator |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
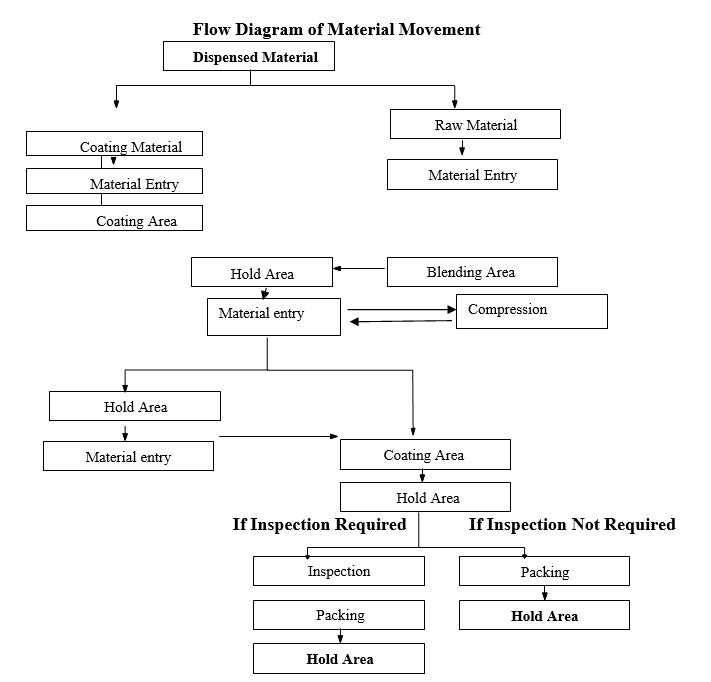
Flow Diagram of Material
ANNEXURE-I
PRODUCT LABEL

ANNEXURE-II
LOOSE PRODUCT LABEL