- OBJECTIVE:
- To lay down the procedure for Testing and Release of In process and Finished Products.
- SCOPE:
This SOP is applicable to the procedure for testing and release of in process and Finished Products at {Company Name} {Location}.
- RESPONSIBILITY:
- Chemist/Executive/Designee QC: Responsible for testing of In process and finished products.
- Head QC or his designee: Responsible for Work Allocation and approval of Analytical data and Responsible for release of In process and finished products.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- Testing and Release of In process samples:
- The Quality Assurance Officer/Designee shall sample the In-process products and submit to Quality Control Department with duly filled sample test request form.
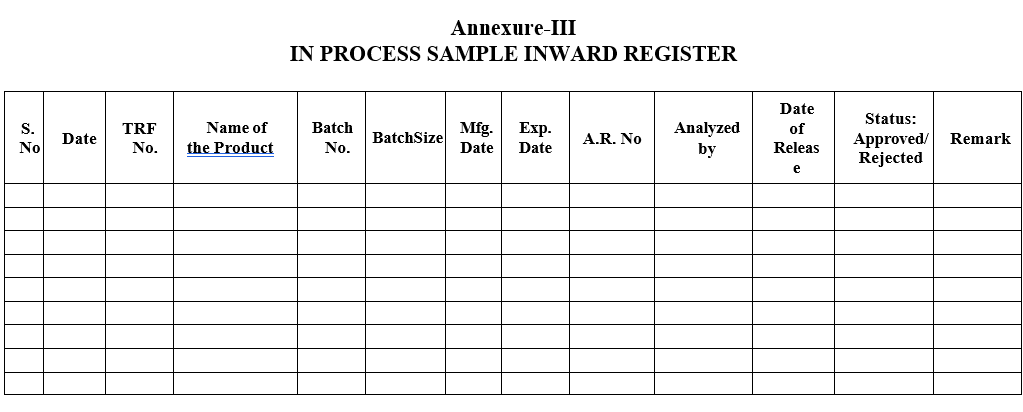
- The Quality Control chemist/Designee shall register the same in In Process Sample Inward Register.
- Executive-QC/Designee shall verify the details on the sample pack and test request form.
- In case of any discrepancy observed, he/she shall immediately inform to QC Manager and QC Manager in turn call the attention of QA Manager and get the test request form/detail on the samples corrected, initiated by Production Manager and counter signed by QA Manager.
- Allot an Analytical report number for the product as per SOP.
- QC Executive/Designee shall allot/delegate the sample to concerned chemists.
- Chemist shall Prepare relevant work sheets as per SOP.
- Analyst shall perform the tests as per Standard Testing Procedure of respective product.
- The chemist shall enter the data in the work sheet, and completed worksheet along with chromatograms or relevant data shall be handed over to Sr. Executive-QC.
- On entry of the results, in case of an OOS result observation, the chemist shall report the same to the immediate supervisor; the supervisor shall initiate the investigation along with the Head-QC as per SOP.
- Sr. Executive-QC/Designee shall review the work sheet as per SOP.
- Section In charge or his designee shall ensure that all the tests are performed as per the standard test procedure and results are reported as per the specification and then only he/she shall enter the Status column with sign in the In Process Sample Inward Register.
- Sr. Executive-QC/Designee shall take two copies of COA’s as per SOP, following the rounding off the digits as per SOP (1st copy for QC compilation and 2nd copy for Production).
- QC Manager or his designee shall ensure that all the tests are performed as per the standard test procedure and results are reported as per the specification and then only he shall sign and date in the “Reviewed by” column in the COA.
- The analytical report along with supporting data shall be submitted to the QA department for verification and QA manager shall sign and date in the “Approved By” column in COA.
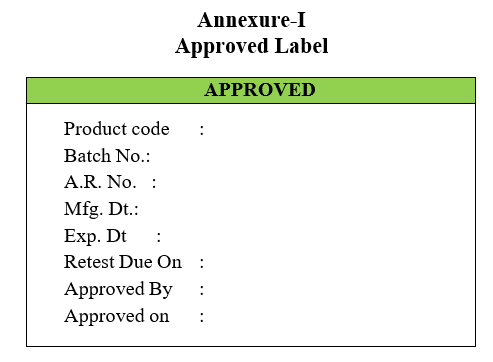
- Quality control in-charge shall approve and sends dually filled Approved Labels to Quality Assurance Department.
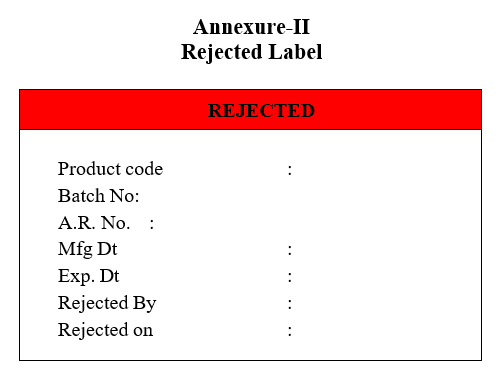
- (‘Rejected Label’ in case the sample fails as per the specification).Quality Assurance Officer/Designee will cross check the report and affix the Approved Slips and clears the in-process product for the next activity.
- Testing and Release of Finished Products:
- Finished products shall be sampled by Quality Assurance personnel and issued to Quality Control department with duly filled sample test request form.
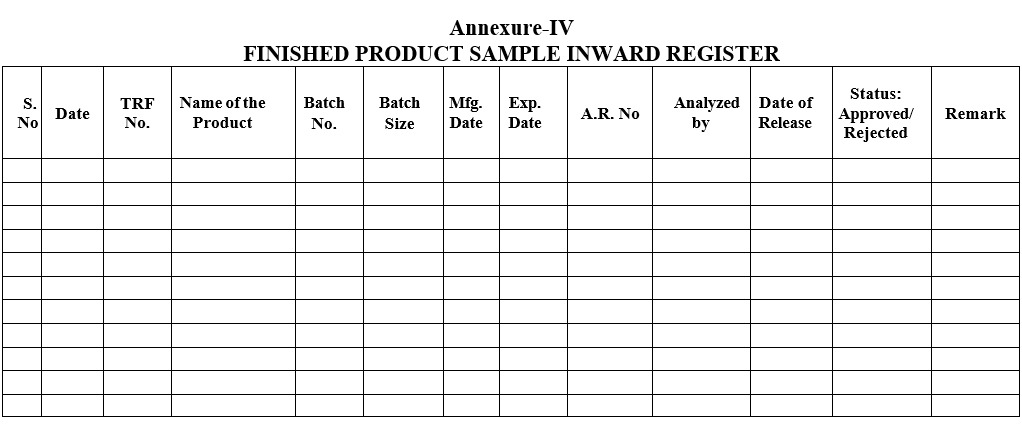
- The details of the product shall be entered in the Finished Product sample Inward Register by QC.
- Executive-QC/Designee shall verify the details on the sample pack and test request form. In case of any discrepancy observed, he/she shall immediately inform to QC Manager and QC Manager in turn call the attention of QA Manager and get the test request form/detail on the samples corrected, initiated by Production Manager and counter signed by QA Manager.
- Allot an Analytical report number for the product as per SOP.
- Chemist shall Prepare relevant work sheets as per SOP.Analyst shall perform the tests as per Standard Testing Procedure of respective productIn charge or his designee shall ensure that all the tests are performed as per the standard test procedure and results are reported as per the specification and then only he shall enter the Status column with sign in the Finished Product Sample Inward Register.
- Sr. Executive/Designee QC shall take three copies of COA’s as per SOP, following the rounding off the digits as per SOP.1 st copy for QC compilation, 2 nd copy for Production and third one for logistic copy.
- QC Manager or his designee shall ensure that all the tests are performed as per the standard test procedure and results are reported as per the specification and then only he shall sign and date in the “Reviewed by” column in the COA.
- The analytical report along with supporting data shall be submitted to the QA department for verification and QA manager shall sign and date in the “Approved By” column in COA.
- Quality Assurance Officer/Designee will cross check the report and affix the Approved Slips and clears the finished product for the next activity.
- If the product fails to meet the specification, then NCR shall be raised and proper investigation shall be carried out as per SOP.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/testing-and-release-of-inprocess-and-finished-products/
- REFERENCES:
Not Applicable
- ANNEXURES:
| Annexure No | Title of Annexure |
| Annexure-I | Approved label |
| Annexure-II | Rejected Label |
| Annexure-III | In Process Sample Inward Register |
| Annexure-IV | Finished Product Sample Inward Register |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| OOS | : | Out of specification |
| SOP | : | Standard Operating Procedure |
| COA | : | Certificate of Analysis |
| QC | : | Quality Control |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
Approved Label
Annexure-II
Rejected Label
Annexure-III
IN PROCESS SAMPLE INWARD REGISTER
Annexure-IV
FINISHED PRODUCT SAMPLE INWARD REGISTER
Frequently Asked Question ?
Q: Who submits the in-process samples?
A: Quality Assurance Officer/Designee with a completed test request form.
Q: Who registers the samples and manages discrepancies?
A: Quality Control chemist/Designee, notifying managers for corrections initiated by Production and countersigned by QA.
Q: Who approves and releases the in-process product?
A: Section In-charge ensures test adherence to SOP and specifications, then signs the register. Sr. Executive-QC issues two COA copies (QC & Production). QC Manager, then QA Manager, reviews and approves the COA. Finally, QC in-charge sends approved labels (rejected if failed) to QA for final release.
Q: Who samples and submits finished products?
A: Quality Assurance personnel with a completed test request form.
Q: Who manages discrepancies and releases samples?
A: QC registers the product, and Executive-QC manages discrepancies like in-process samples.
Q: Who approves and releases the finished product?
A: Section In-charge ensures test adherence, Sr. Executive-QC issues three COA copies (QC, Production, & Logistics). QC Manager and QA Manager review and approve COA. QA confirms the report, affixes approved slip, and clears the product for next activity. Failure triggers NCR and investigation per SOP.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/testing-and-release-of-inprocess-and-finished-products/
