OBJECTIVE:
To lay down a Procedure for Training of Personnel.
- SCOPE:
This SOP is applicable to the Training of all the Personnel engaged in Manufacturing, Testing, Packing, Holding and Distribution of Pharmaceutical Products and also engaged in cGMP Training and other as identified for effective and Control Operation at {Company Name} {Company Location}.
- RESPONSIBILITY:
HR Training Co-ordinator shall be responsible for specimen signature, GDP & induction Training.
Training Co-ordinator QA or designee shall provide cGMP Training Module. He /She shall co-ordinate with the cross functional department for completion of training as per Training Need Identification. He/She shall also be responsible to co-ordinate for the cGMP trainings of the New Entrants.
Departmental Training Co-ordinator shall be responsible for job specific training and storage & maintenance of individual training records.
Concern Department Head shall be responsible for the completion of training of all personnel and ensuring the completion of individual training records.
Individual shall be responsible for maintaining their respective training records.
- ACCOUNTABILITY:
Head Quality Assurance shall be accountable for implementation & compliance of this SOP.
- PROCEDURE:
Every Employee of the organisation shall be trained on his/her area of operation prior to start the work. The Training shall be depended on the nature of job and responsibilities and the cGMP training is mandatory for each Employee.
- SELECTION OF TRAINER:
- Head QA in consultation with respective Department Head shall identify Internal Trainer from different Department based on his Qualification, Skills, Experience, Knowledge, Communication and Expertise in the different areas of Operation.
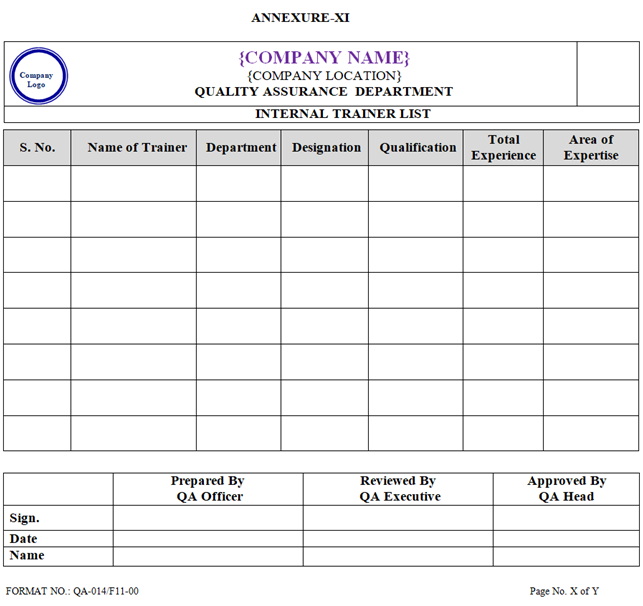
- A list of Internal Trainer shall be prepared by QA Department as per Annexure-XI, reviewed by Assist. Head QA/designee & approved by Head QA.
- The list of Internal Trainer (Controlled Copy) shall be circulated to each Department Head for reference.
- Head QA shall be evaluate the technical competency of trainer on the following points:
- Technical Education Qualification
- Work Experience
- Exposure to external trainings
- Exposure to Regulatory Audits
- Trainer shall be also evaluated by the means of a verbal feedback from trainees after training session. If training of any trainer not found satisfactory, Head QA has right to replace him / her as trainer.
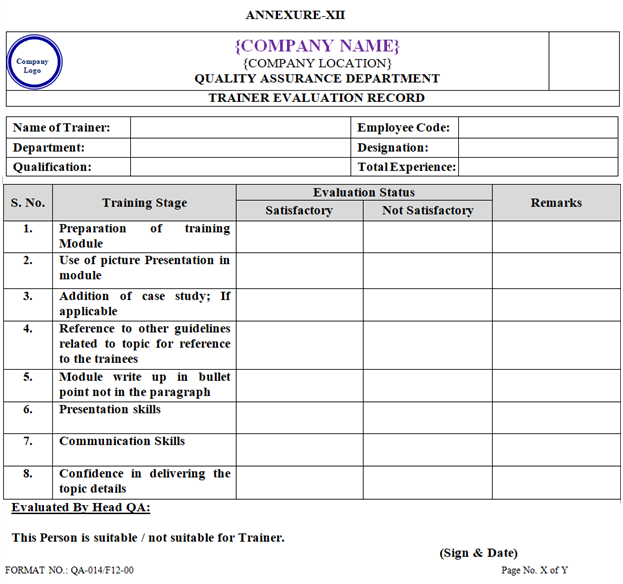
- Trainer evaluation record shall be prepared as per Annexure-XII.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/training-of-personnel/
- CERTIFICATION OF TRAINER:
- After evaluation individual shall be allowed to work as a Trainer and Training Certificate as per Annexure-XIII shall be given to him / her.
- In case, Head Operations act as a trainer their Trainer Certificate shall be signed by Head QA.
- In case, Head QA act as a trainer their Trainer Certificate shall be signed by Director of {Company Name}
- TRAINING PLAN:
- Training Plan shall be prepared in such a way that all the regulatory and in-house aspects shall be Covered and documented properly for the successful completion of the Training.
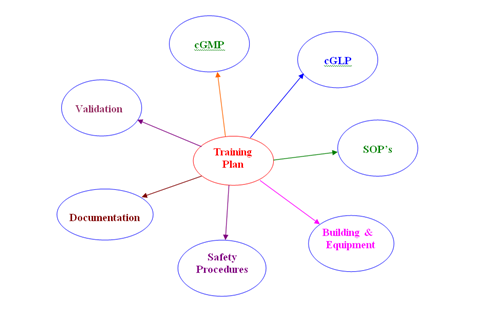
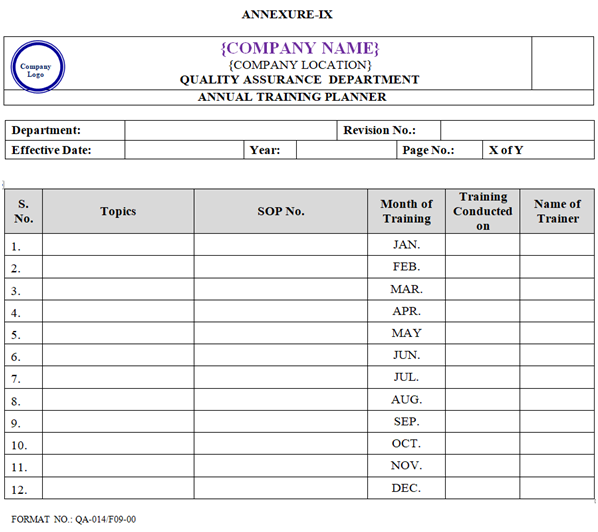
- An Annual Training Planner shall be prepared in the beginning of each Year. This Planner acts as a tool for providing various training programs which are required for the improvement of the Technical skill of the Personnel.
- The Training Committee (Consist of all department Training Co-ordinator) shall be constituted for the execution of these Training Programs. Members of this committee shall be drawn from Production, QC, Engineering, Warehouse, Human Resource, EHS and QA.
- The members can be Head of the Department or anybody nominated by them. However in the light of recent advancements in the Regulatory Requirements, this Training Planner is subjected to revision if required.
- Intimation of Training shall be initiated by QA.
- One week prior to execution of cGMP and Technical Training the Quality Assurance Department shall circulate a reminder to concerned Department Head, so that they can plan accordingly the activities of their Employees.
- Concerned Department in Co-ordination with QA shall send communication to different Department Head about the Date, Time and Place of Training Session (in case of concern topic is cross functional).
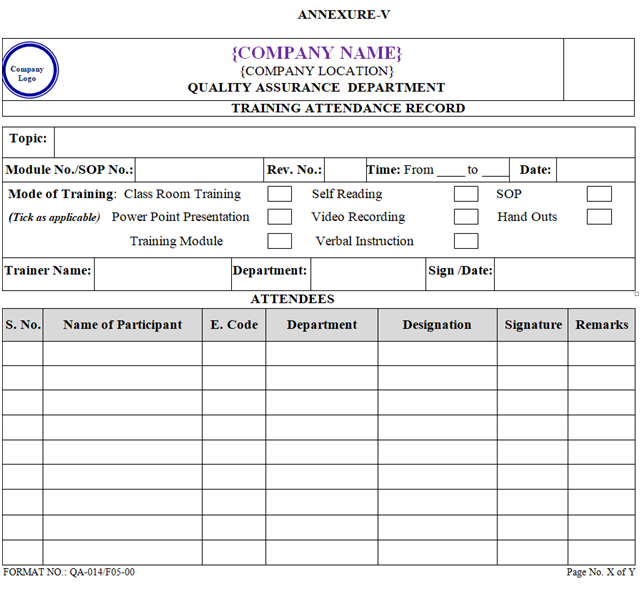
- Prior to start the training the participant shall fill their attendance in training Attendance Record as per Annexure–V.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/training-of-personnel/
- TRAINING MODULE:
- The technical or cGMP related topics shall be conducted in the form of PPT Training.
- Trainer shall prepare the Training Module on relevant topics in Presentation Styles and the same shall be reviewed by Training Committee.
- Trainer shall deliver the content as per Training Module but not limited to Particular Topics.
- TYPES OF TRAINING:
The types of training are divided into two Major Categories as Internal Training and External Training.
- INTERNAL TRAINING:
Internal Training is the Training which shall be conducted within the Organisation by the In house Qualified Trainer who have been certified and trained to impart effective training. It is also divided in to six main categories as mentioned below:
- Induction Training
- cGMP / Technical Training
- Job Specific Training (Training of New Joinee before Execution of Work)
- On Job Training
- Refresher Training
- Re-Training/Need Based Training
- Induction Training:
- Training, which is covering the overview of Organisation, Parent Department and Cross Functional Departments, shall be defined as Induction Training.
- The Induction Training shall be imparted to every new employee, who joins the Organisation and shall be co-ordinated by Head HR or his / her designee.
- The induction should be completed in minimum one working day, starting from the date of joining.
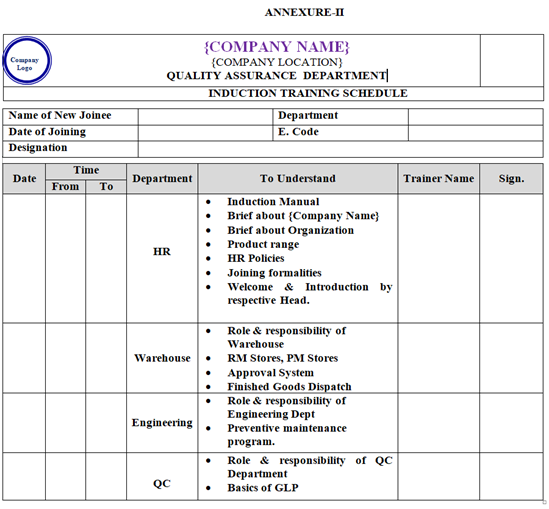
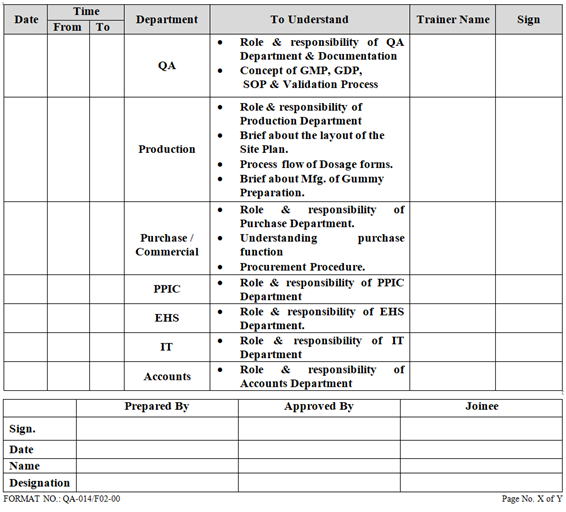
- HR Department shall prepare an induction training schedule for every new joinee and communicate induction schedule to all department through verbal or telephonic/mail as per Annexure–II.
- Initial Induction Training is carried out by Head HR or his / her designee. Induction training manual shall be used by HR Personnel for the training purpose which cover topics like:
- Introduction about {Company Name}
- Organisational Structure.
- Entry & Exit into the factory premises
- Key Personnel and different Department Structure and details.
- Different Product Range.
- HR Policy’s w.r.t Shift Timing, Leave Policy and other Employee Benefit Policies.
- HR Department facilities w.r.t. Canteen, Transportation, Medical etc.
- Security system and safety measures.
- The new entrant shall visit to respective department as per schedule and training (about departmental structure, departmental functions and cross functional areas) shall be given by department Head/ designee.
- Job Specific Training (Training of New Joinee Before Execution of Work)
- Job responsibilities shall be assigned to new entrant as per the respective SOP.
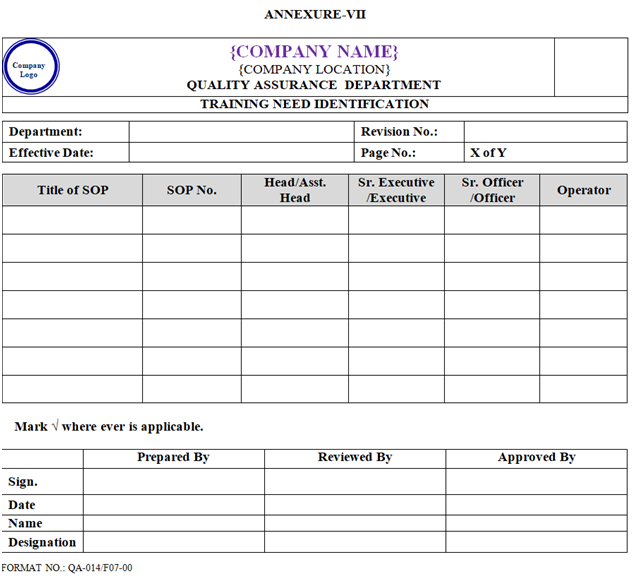
- Concerned department Head/designee shall plan training of new Joinee on the basis of job Responsibility and training need identification as per Annexure-VII.
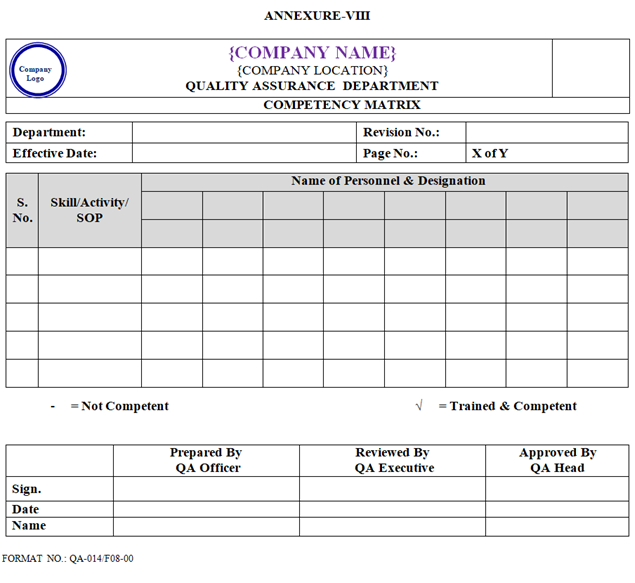
- As per work requirement and competency, competency matrix shall be prepared by concerned department Head/designee and approved by QA Head as per Annexure-VIII.
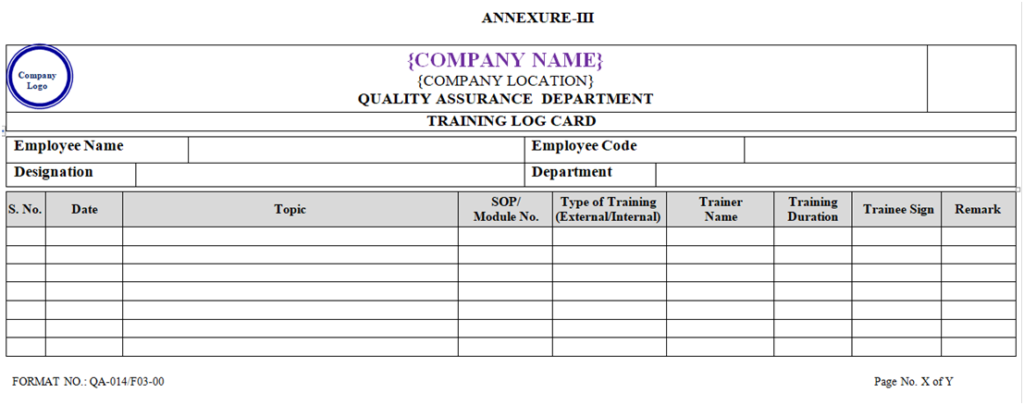
- Trainee shall record the details of training in their respective training log card as per the Annexure-III.
- All new entrants joining organisation at designation of Assistant Head and above shall undergo the self training and no evaluation is required.
- After the completion of the training program, New Entrants shall be evaluated by the respective department Head (in case of new entrant is respective department Head shall evaluated by QA Head) and certificate of completion of training shall be issued as per the Annexure-X.
- In case of all plant personnel, Certificate of training to new Entrants shall be certified by Head QA as per Annexure-X after successful completion of the training program.
- In case of Head QA, Certificate of training to new Entrant shall be certified by Managing Director as per Annexure-X after successful completion of the training program.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/training-of-personnel/
- cGMP / Technical Training:
- cGMP Training covers the various aspects covered as per different Regulatory Guidelines of cGMP. The purpose of cGMP is to ensure that laid down system is adhered by personnel in the Organization.
- Technical Training is the Training which is designed to develop specific and cross specific skill and enhance the Technical Knowledge.
- The Quality Assurance Department with concerned of different Department Head shall prepare a Annual Training Planner as per Annexure-IX and Technical Training at the start of every Calendar Year.
- The Internal cGMP / Technical Training shall be conducted at least once in Year and as when identified by certified Trainer. Technical training like: cGMP, GDP, GLP, Cross-Contamination, Sanitization & Personnel Hygiene, basic EHS awareness etc.
- cGMP training shall be mandatory for the new joinee prior to put into the area of operation.
- The cGMP Training shall be conducted by Trainer as Class Room training by the means of Power Point Slide Presentation or Module.
- The scope of cGMP / Technical Training includes aspects like as follows but not limited to the followings only:
- Basic cGMP aspects of Different Regulatory Agency.
- Any revision / updation to Regulatory Guidelines etc.
- Topics cover safety aspects of the persons engaged in Manufacturing Operation.
- Other as identified during the routine Operation.
- The Training material used by the Trainer shall be distributed after the training to the employees attended the Training Programme if required.
- In addition, the websites of the Regulatory Agencies (such as MHRA, EU GMP, USFDA, ANVISA etc) shall be referred by QA to gather the information for changes in the GMP regulations, audit procedures, submission requirement, variation application requirements, risk assessment etc. This tracking shall be done and circulated once to all concern departments and to operations for quality system enhancements.
- On the Job Training:
- The functional Heads/ Designee (Ttraining Co-ordinator) shall prepare an Annual Training Planner (covering departmental SOPs, Cross functional department SOPs cGMP/Technical Training) on annual basis as per Annexure-IX and these planner shall be approved by Head QA.
- One copy of Annual Training Planner shall be distributed to concern department for execution. Master copy of same document shall be retain in quality assurance department.
- If a particular training has not been conducted as per the training Planner or schedule, department Head/designee shall inform to QA department for the same and shall arrange to conduct the training within one working week.
- Attendance of each training session shall be recorded in training attendance record as per Annexure-V respectively and shall be maintained by concerned department.
- All plant personnel shall attend the training according to SOP as per Annual Training Planner and same shall be record in Log Card as per Annexure-III.
- All plant personnel shall attend the minimum training required as per competency matrix and as required for their Job Responsibility.
- All Department Head/functional Head ensure that each personnel should attend training of all job related SOPs at least once in a year.
- Training of the required SOP and other related areas to the Operator / Worker shall be given by the concerned area in charge (Trainer). The Training of SOP for the worker shall be on the local regional language so that it can be easily understand by them.
- If any Employee is transferred from one area of operation to other area of operation within the department then the related SOP Training shall be given to the concerned employee.
- Refresher Training:
- All employees shall undergo Refresher Training on the procedures of their respective functions and related processes or cross functional department. Wherever applicable.
- Refresher Training shall be carried out once in a year and whenever there is a Procedural Change or Revision before Implementation.
- Re-Training / Need Based Training:
- Re-Training can be given on any Technical Topic and cGMP as and when required or identified by the Concerned Head.
- If during any type of Training, the evaluation marks are less than 80% than the employee shall be retrained.
- Need based training shall be conducted in reference to the additional requirement of incident/deviation/OOS/ Market Complaint, OOT, Self Audit, External Audits, Performance reviews and behavioural issue etc.
- This is required for the specific persons, so the reoccurrence of incident, Deviation, OOS, Market complaint, OOT and behavioural issue occurring due to the personnel error can be prevented.
- The details of the training shall be recorded in training Attendance Record as per Annexure-V and also record in Log card as per Annexure-III.
- External Training:
- The external cGMP / Technical training shall be conducted at least once in a year or as and when required.
- The external training programme shall be identified by the concerned Department Head and information shall be sent to Head QA and HR.
- Selection of outside training programme and identification of the employees for training shall be done by Concerned Department Head and Head QA.
- After attending the External Training Programme at Outside, Trained Employee shall conduct the Training Programme on the same topic on which he / she is being Trained.
- Department Head may ask the concerned employee to make a presentation on the topic concerned for the benefit of all those concerned who were not sent for training.
- The selection of External Trainer for In house Training shall be done by the concerned Department Head and Head QA. The selection of Trainer shall be primarily based on his Experience, Qualification, Skills and Expertise on the training topics.
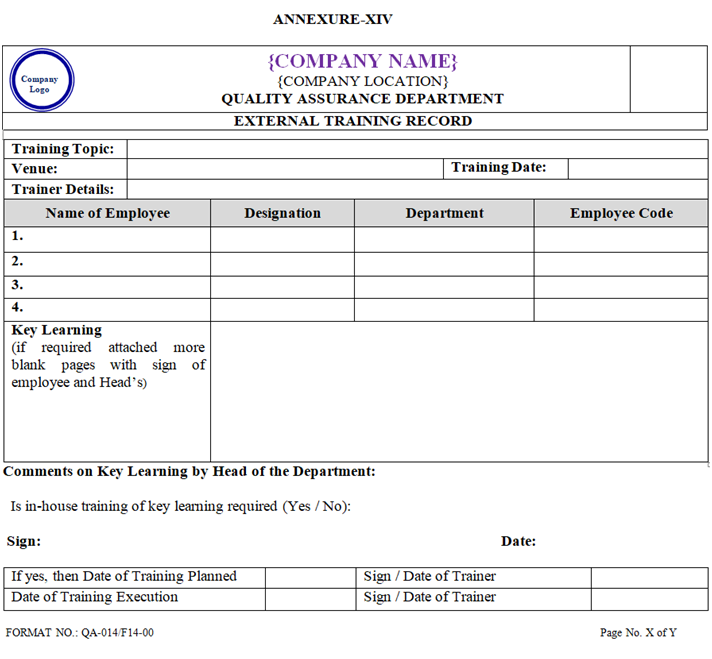
- After attending the Training Programme at outside it shall be recorded in the External Training Record shown in Annexure-XIV.
- Maintain the training record in training file of concerned employees who have undergone external training.
- TRAINING NEEDS IDENTIFICATION:
- Each functional Headshall identify the training needs of the personnel based on their job role and responsibilities. Such training may be on the SOPs, GMP and other cross functional requirements. The same shall be submitted to QA for approval of Training Need Identification as per Annexure-VII.
- Competency Matrix shall be prepared and updated by respective Department as per Annexure-VIII.
- There may be need to train the personnel through demonstration at his/her work place on specific operation/procedure. Such training shall be conducted by certified Trainer.
- In an event the personnel is assigned with a new job role / responsibility, he /she shall undergo training in the new area e.g. rotation of personnel from packing material analysis to raw material analysis, rotation from manufacturing area to Packing area.
- TRAINING ON NEW SOP/ REVISED SOP:
- Training on new SOP/revised SOP shall be given by concern department Head/designee to all concern employees after approval and before implementations of SOP. Whenever SOP revised training on revised SOP shall also be carried out.
- Whenever a new SOP implemented or existing SOP revised, photocopy of training attendance record as per Annexure-V shall be attached with respective master SOP.
- SELF TRAINING:
- The personnel who have adequate knowledge /experience in a particular area, may be requested to understand the procedures and SOPs and relevant practices in the organization by going through SMF, VMP, manuals, and qualifications documents etc. Such trainings shall be under self trainings program and shall be applicable to assistant Head and above.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/training-of-personnel/
- TRAINING RE-SCHEDULE:
- In case of Failure in adhering to the scheduled Training Calendar (Annual Training Planner), the same shall be conducted within week:
- If any employee is absent, training on remaining topic to him / her shall be imparted in Last week of the month.
- TRAINING SHALL BE IMPARTED IN ONE OF THE FOLLOWING WAYS:
- Classroom Training:
- Itis a conventional method of training in which the trainer by virtue of knowledge and expertise will impart the training on the subjects, Quality System, Standard Operating Procedures, Corporate guidelines, General Testing Procedures, Good documentation practices, Warehousing Practices etc. Power point presentation, video visuals may be used for such training.
- Interactive and participative training:
- Includes seminars, workshops, conferences, presentations, demonstrations, lectures, case studies, group discussions, group training programmes.
- The personnel may be asked to read the manual or SOP and understand the procedure.
- The training can also be imparted by verbal instruction to the personnel to make him understand the procedure.
- Handouts /Modules /write ups /guideline, may be used for training of personnel.
- EVALUATION OF TRAINING:
- Once the training has been completed by the employee, he/she shall be evaluated to know the extent of his/her understanding.
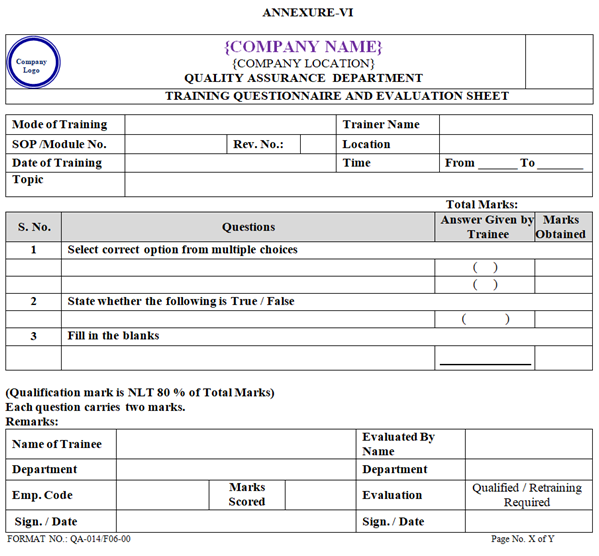
- The Evaluation shall be conducted for each type of Training by the means of Questionnaire or Oral Feed Back on need basis to check the effectiveness of Training as per Annexure-VI.
- Note: The trainees who fail to understand the training course shall not fill the questionnaire and shall require retraining. Also, in case of workers or operators who are unable to understand the English, the entire questions should be translated in Hindi or local language.
- The Questionnaire shall include Questions related to each aspect of Training Topic and the Questions can be filling the blanks, true/false and Multiple choice.
- The filled questionnaires of respective department Training Co-ordinator shall be evaluated by trainer whoever giving the training.
- Finally the filled questionnaires shall be evaluated by respective department Training Co-ordinator based on set of questionnaire.
- If the score mark is less than 80% then the Employee shall be retrained & re-evaluated immediate after evaluation.
- There could be minor amendments in the instruction in SOP which can be passed on by verbal instruction /demonstration by qualified trainers and need not to be assessed through training evaluation programme (questionnaire).
- The personnel reviewing and approving the SOPs shall be considered trained on that particular SOP and no need to maintain separate training records.
- During evaluation of questionnaire, if trainee scores more than 80% and less than 100% then all correct answer (which the trainee unable to answer correctly) shall be explained immediately after evaluation to makes sure that he/she understand the SOP/ training topic 100% and put the comments in remark on questionnaire for the understanding of topic with signature of trainee and trainer, If the trainee fails to qualify than retraining shall be given immediate after evaluation.
- DOCUMENTATION:
- The documentation of different type of Training shall be done by the Departments as mentioned below:
| Induction Training | : | HR Department |
| Job Specific Training (Training of new Joinee before execution of work) | : | Concerned Department |
| On the Job Training | : | Concerned Department |
| cGMP / Technical Training | : | Quality Assurance Department |
| Refresher Training | : | Concerned Department |
| Re-training/ Need based Training | : | Respective department |
| External Training | : | Quality Assurance Department |
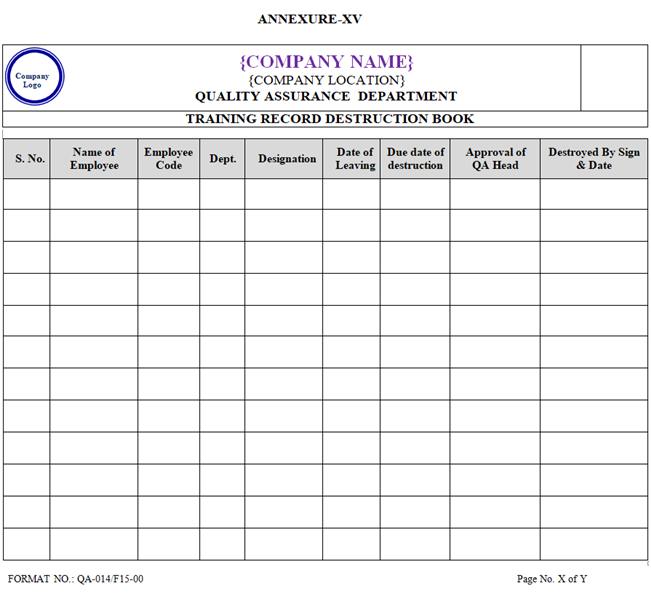
- If any employee leaves the organisation then his / her training records (Training File) shall be maintained for a period of not less than Two year from the date of relieving by the HR Department. Subsequently the records after Two year shall be destroyed by QA department and shall be recorded in Training Destruction Log Book as per Annexure-XV.
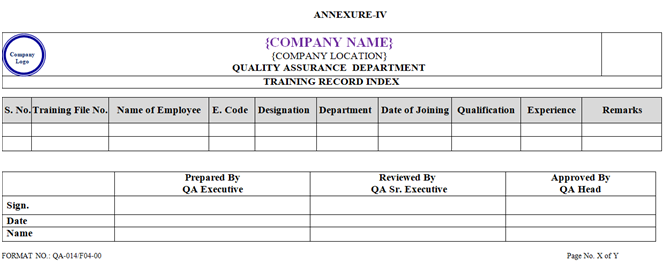
- Relieving date of employee who has resigned shall be written in remark column in Training record index by HR as per Annexure-IV.
- The Quality Assurance shall be responsible for verification of the training status and records.
- All Training Record shall be compiled in Yellow Colour “Training Record” file.
- Format for “Training Record” cover file shown in Annexure-I and format no. printed only on front cover.
- Training Record Index shall be prepared by Quality Assurance Department as per format shown in Annexure-IV and shall be updated by HR Department after every Three Month or whenever required.
- After completion of year all training records shall be submitted to QA department for future reference.
- In case of any employee left from company, training record of same shall be discontinued by concerned department Head and record of such employee shall submitted to QA department along with their Job responsibility.
- All employees are responsible to update their respective training record.
- The individual training files of all employees shall be kept under control of training Co-ordinator from respective department.
- List of training Co-ordinator shall be prepared by QA Officer / executive and approved by QA Head as per Annexure-XVI. Name of Training Co-ordinator shall be nominated by respective department Head.
- HR Department shall issue the Training File to the new entrant.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/training-of-personnel/
- TRAINING FILE NUMBERING SYSTEM:
- Each Training File shall be issued by HR Dept. given a Unique Number, once number is allotted to any Training File; the same number shall not be assigned to any other Training File.
- All Training File shall contain Seven Alphanumeric Characters (Two Alphabets, Four Numeric and One Separator).
- For Example the First Training File shall be numbered as TF/NNNN.
- Where:
| TF | : | Indicate the code of Training File |
| ‘/’ | : | Is Separator |
| NNNN | : | Indicate the Serial Number of Training File |
3rd character ‘/’ is Separator and 4th, 5th, 6th & 7th characters are numeric digits ‘NNNN’ indicating the Serial Number of Training File.
- REFERENCES:
Training Manual.
ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE | FORMAT No. |
| Annexure-I | Training Record Cover File | QA-014/F01-00 |
| Annexure-II | Induction Training Schedule | QA-014/F02-00 |
| Annexure-III | Training Log Card | QA-014/F03-00 |
| Annexure-IV | Training Record Index | QA-014/F04-00 |
| Annexure-V | Training Attendance Record | QA-014/F05-00 |
| Annexure-VI | Training Questionnaire and Evaluation Sheet | QA-014/F06-00 |
| Annexure-VII | Training Need Identification Matrix | QA-014/F07-00 |
| Annexure-VIII | Competency Matrix | QA-014/F08-00 |
| Annexure-IX | Annual Training Planner | QA-014/F09-00 |
| Annexure-X | Certificate of completion of training of New Entrants | QA-014/F10-00 |
| Annexure-XI | Internal Trainer List | QA-014/F11-00 |
| Annexure-XII | Trainer Evaluation Record | QA-014/F12-00 |
| Annexure-XIII | Certificate of Qualified Trainer | QA-014/F13-00 |
| Annexure-XIV | External Training Record | QA-014/F14-00 |
| Annexure-XV | Training Destruction Log Book | QA-014/F15-00 |
| Annexure-XVI | List of Training Co-ordinator | QA-014/F16-00 |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
| Controlled Copy No. 01 | : | Head Quality Assurance |
| Controlled Copy No. 02 | : | Head Quality Control |
| Controlled Copy No. 03 | : | Head Warehouse |
| Controlled Copy No. 04 | : | Head Production |
| Controlled Copy No. 05 | : | Head Engineering |
| Controlled Copy No. 06 | : | Head HR |
| Master Copy | : | Quality Assurance Department |
ABBREVIATIONS:
| SOP | : | Standard Operating Procedure |
| No. | : | Number |
| Ltd. | : | Limited |
| Pvt. | : | Privet |
| QA | : | Quality Assurance |
| Dept. | : | Department |
| w.r.t. | : | With respect to |
| HR | : | Human Resource |
| cGMP | : | Current Good Manufacturing Practices |
| S. No. | : | Serial Number |
| QC | : | Quality Control |
| RM | : | Raw Material |
| PM | : | Packing Material |
| PPIC | : | Production Planning and Inventory Control |
| EHS | : | Environment Health and Safety |
| IT | : | Information Technology |
| GLP | : | Good Laboratory Practices |
| Assist | : | Assistant |
| MHRA | : | Medicines and Health care products Regulatory Agency |
| EU GMP | : | European Medicine Agency |
| USFDA | : | United State Food and Drug Administration |
| ANVISA | : | Agencia Nacional de Vigilancia Sanitaria |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
ANNEXURE – I


Click the link for download word file copy of this document: https://pharmaguidehub.com/product/training-of-personnel/
ANNEXURE-II
ANNEXURE- III
ANNEXURE – IV
ANNEXURE – V
ANNEXURE – VI
ANNEXURE – VII
ANNEXURE VIII
ANNEXURE – IX
ANNEXURE – X

ANNEXURE – XI
ANNEXURE XII
ANNEXURE – XIII

ANNEXURE – XIV
ANNEXURE-XV
ANNEXURE-XVI
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/training-of-personnel/