OBJECTIVE:
To describe the procedure for trending and evaluating manufacturing process parameters and analytical test results.
SCOPE:
This procedure is applicable to the drug products manufactured for commercial distribution at {Company Name} {Location}.
RESPONSIBILITY:
Quality Assurance: For collection of process parameters and analytical results of commercial batches and trending. Preparation and review of Quarterly trend report for each product. Identify the Corrective and Preventive Action based on product quarterly trends. Maintenance of Quarterly trend reports.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
PROCEDURE:
Control chart shall be used (Using excel spread sheet) to perform the trend analysis of process parameters and analytical test results, which are critical quality attributes.
At least 6 independent data points are required to initiate and to calculate control limits. Preferably, at least 40 independent data points are required to derive the permanent control limits.
Data shall be included in the chart for all commercial products upon the availability of data.
For each quarter of the year, a trend report shall be obtained from the excel file and shall be compiled for each product.
If there is any out of trend observed in the quarterly trend the same shall be investigated and appropriate corrective and preventive action shall be taken.
Calculating control limits: Complete the following steps to establish control chart limits:
Enter the individual results in excel spread sheet.
When at least 6 results are available, calculate UCL and LCL (3- sigma control limits).
The formula for calculating the Upper Control Limit (UCL) and LCL (Lower Control Limit) are given below,
Upper Control Limit: X + 3 σ
Lower Control Limit: X – 3 σ
Where,
X- Sample Mean – Process Average
S – Standard deviation (σ)
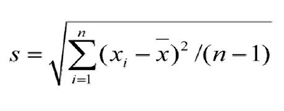
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/trend-analysis/
Determination of “Temporary” or “Permanent” control limits.
| Number of data points | Additional requirements (when the data points are plotted on the control chart) | Type of control chart limit |
| Less than 6 | Not Applicable | Limits cannot be determined |
| Less than 6 but less than 40 | Not Applicable | Temporary |
| At least 40 | If the trending of data shows any of the either criteria, There are more than 5 consecutive or 9 per every 40 points beyond 3 sigma | Temporary |
| If the trending of data shows no such deviation as mentioned above | Permanent |
If in a run, there are more than 5 consecutive data results or 9 data results beyond 3 sigma, within 40 batch data are observed.
- Investigate the cause of this specific excursion through Failure investigation procedure.
- Process changes are to be verified.
- If the cause is identified, remove those results for control limit calculation/ trend.
- Impact of these excursions on the product quality shall be evaluated, as applicable.
- If the cause is not identified, use all the results for control limit calculation/ trend.
Out of trend in the process is defined if:
- Five or more similar observations within ten consecutive batches beyond 3 sigma value from the permanent control limit.
- More than nine similar observations batches beyond 3 sigma value from the permanent control limit in the subject PQR review period.
When out of trends is identified during trend
- Investigate the cause of this specific excursion through Failure investigation procedure.
- Determine the appropriate corrective and preventive actions needed to address the observations by discussing Production, Process development and Formulation Research and Development, as applicable.
- Impact of these excursions on the product quality shall be evaluated and concluded in the investigation report.
REFERENCES:
21 CFR 211.180
USP-33 – General chapters 1010 and 1080
ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
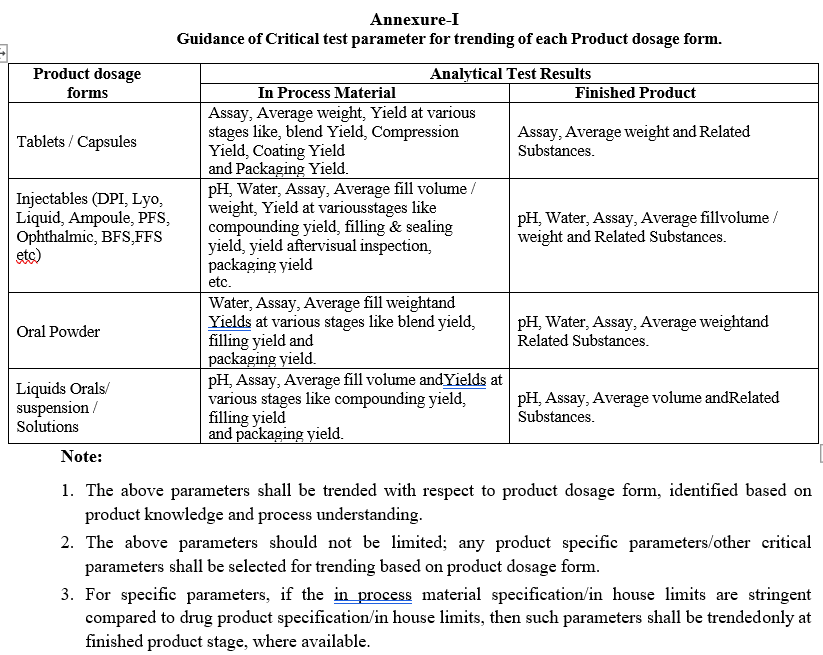
| Annexure-I | Guidance of Critical test parameter for trending of each Product dosage form |
ENCLOSURES: SOP Training Record.
ABBREVIATIONS:
| PD | : | Production |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| CAPA | : | Corrective And Preventive Actions |
| OOS | : | Out of Specification |
| QMS | : | Quality management system |
Definitions:
Control chart: A line graph used to display the results of repeated test results/process parameters over time. A control chart has limits statistically established from historical data from the given process. A control chart is a tool that assists in monitoring the process performance and detection of trends.
Control chart limits: Statistical limits established from historical data from a given process. The width of these limits represents the natural, common-cause variability of the process. They are set by default at 3-sigma from the process average. Control Chart Limits are defined limits to be taken into consideration during a given process review.
Process out-of-control: Process whose value exhibits points either beyond the control chart limits or with a non-random pattern (e.g. trend). An out-of-control process contains special-cause variation, thus it is not stable or predictable.
Permanent control chart limits: Control chart limits created from a sufficient amount of stable and predictable data.
Temporary control chart limits: Control chart limits created from a limited amount of data (less than 20), or created from unstable data.
Trend: The process by which data and/or information gathered through tracking is evaluated for the general and/or specific tendencies over a course of time to determine whether positive or negative changes has occurred or could occur.
Critical Quality Attributes (Critical test parameter) is a physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality.
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
Guidance of Critical test parameter for trending of each Product dosage form.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/trend-analysis/