OBJECTIVE:
To lay down the procedure for Usage and transfer of Purified Water in production area.
SCOPE:
The scope of this SOP is for the Usage and transfer of Purified Water in the production area.
RESPONSIBILITY:
Initiator Executive/Designee: Production shall ensure the compliance of the SOP.
Initiator Officer/Designee: Production shall perform the operation activity as per SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
About Purified water:
Purified water plays a vital role in various pharmaceutical processes. It serves as a crucial ingredient in the formulation of many non-sterile pharmaceutical products, including tablets, capsules, and topical medications. Additionally, purified water is extensively used as a solvent in the synthesis of active pharmaceutical ingredients (APIs) and intermediates. It also finds application in the cleaning and rinsing of pharmaceutical equipment and product-contact components, ensuring the removal of impurities and maintaining product integrity.
PROCEDURE:
Use purified water for cleaning the equipments and for batch processing.
Drain away the purified water from the pipe before activity for 2- 3 minutes.
Close the valve after usage of water securely to avoid leakage of water.
If any leakage of water observed after closing valve, intimate the Maintenance department to take corrective action
Transfer of Purified Water from one area to another area:
Area where the purified water loop is not available following procedure should be followed:
Collect the purified water in a cleaned SS container and closed the container with lid.
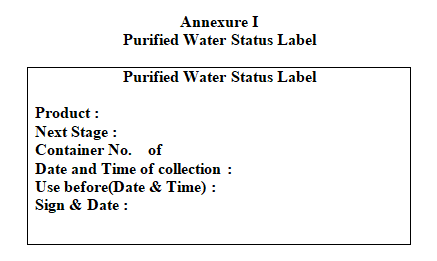
Write purified water status label and affix it on the container.
Stretch wraps the container properly and transfer the container to the required area.
After the collection from user specific point, the purified water should be used within 8 hours.
If purified water is not used within the specified time period, the water shall be drained.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/usage-and-transfer-of-purified-water-in-production-area/
REFERENCES:
Not Applicable
ANNEXURES:
| Annexure No. | Title of Annexure |
| Annexure I | Purified Water Status Label |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
Controlled Copy No. 01 : Head Quality Assurance
Controlled Copy No. 02 : Head Production
Master Copy : Quality Assurance Department
ABBREVIATIONS:
| PD | : | Production |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| SS | : | Stainless steel |
| QA | : | Quality Assurance |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure I
Purified Water Status Label
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/usage-and-transfer-of-purified-water-in-production-area/




