OBJECTIVE:
To define the Validations Policy for manufacturing products of consistent acceptable quality. The extent and degree of validation activity shall depend(s) on the nature and complexity of the equipment/product/process.
SCOPE:
This SOP is applicable for validation approach of new equipments / systems / instruments / processes at {Company Name} {Location}.
RESPONSIBILITY:
Validation Coordinator: Representatives of QA shall be responsible for preparation of validation protocols/reports, preparation of the schedule for conducting the validation studies and summarize and evaluation of validation data. QA in charge or his designee shall be responsible for Review of validation protocols and reports, Monitor the progress to ensure completion of validation as per schedule, to provide resource required for the validation studies within the given schedule.
Validation core committee: It comprises of Head QA, Head Engineering, Head Quality control & Head Production or their designees shall be responsible for Overall co-ordination of the validation programme, Review and Approval of validation protocols /reports, monitor applicable for c GMP aspects and identification of the training needs.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
PROCEDURE:
The facility shall be validated in accordance with the systems, guidelines and procedures established by {Company Name}
All new equipments/systems/instruments/processes introduced in the manufacturing unit shall be validated prior to routine use.
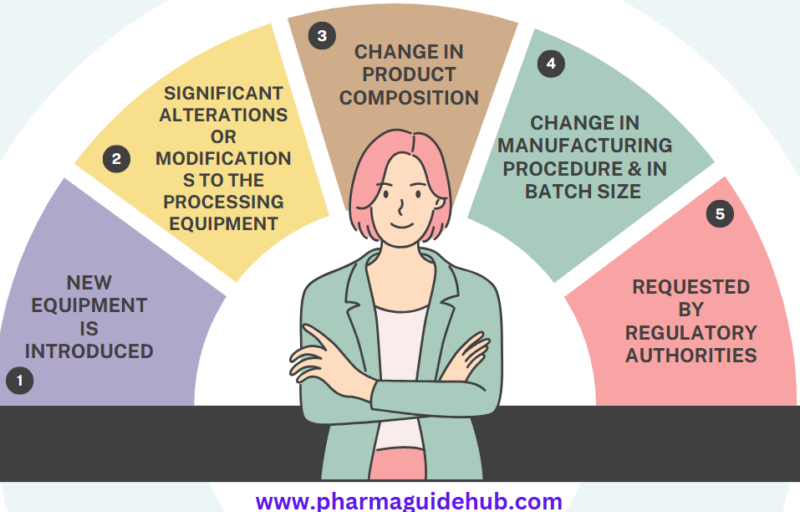
Validation is required for the following, but not limited to:
- If new equipment is introduced.
- In the event of significant alterations or modifications to the processing equipment
- If the composition of the product, the manufacturing procedure, or the batch size is changed.
- If requested by regulatory authorities.
Validation protocols shall be prepared by the validation coordinator as per the contents listed out for each type of validation protocol as per the Validation Master Plan.
Validation protocols shall be checked by the task force leader and approved by the validation core committee.
The Validation Core Committee shall be comprised of senior representatives from the following departments/functions who will manage the validation program.
- Quality Assurance
- Production/QC (as applicable)
- Engineering
The execution team shall be responsible for the execution of the approved protocols.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/validation-policy/
New equipments shall be validated for Design Qualification, Installation Qualification, Operational Qualification and Performance Qualification (as applicable).
Re-validation of equipment, process and method shall be done as defined in the master validation plan.
Cleaning procedures shall be validated for the equipments and accessories used in the manufacturing process.
Analytical methods shall be validated for quantification of active ingredient, degradation substances, and for residual solvents in the product.
The report shall be prepared based on the results obtained.
The report shall be reviewed for approval by the validation core committee.
REFERENCES:
Not Applicable
ANNEXURES:
Not Applicable
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Production
- Controlled Copy No. 03 : Head Quality Control
- Master Copy : Quality Assurance Department
ABBREVIATIONS:
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| QA | : | Quality Assurance |
| QC | : | Quality Control |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/validation-policy/