OBJECTIVE:
To lay down a Procedure for Zero setting of Magnehelic Gauge.
SCOPE:
This SOP is applicable for Zero setting of Magnehelic Gauge at {Company Name} {Location}.
RESPONSIBILITY:
Technician- Follow the instruction as per procedure.
Engineering Officer/Executive- Execution as per laid down procedure.
Engineering Head- Technical correction, review, training & monitoring of SOP.
ACCOUNTABILITY:
Engineering Head
Quality Assurance Head
ABOUT MAGNEULIC GAUGE
In pharmaceutical settings, the Magnehelic gauge plays a vital role in maintaining controlled environments. Its precision is crucial for monitoring differential pressure in cleanrooms, isolators, and laminar flow hoods, ensuring product sterility and preventing contamination. These gauges measure minute pressure differences that indicate airflow and filter integrity. By monitoring these pressures, pharmaceutical facilities can verify proper ventilation and containment, critical for manufacturing sterile products. The gauge’s reliability and accuracy are essential for compliance with stringent regulatory standards, contributing to the overall quality and safety of pharmaceutical production.
PROCEDURE:
Precautions and general conditions:
Ensure that the magnehelic gauge is calibrated and calibration status sticker is affixed on it.
Check the magnehelic gauge for its zero setting as follows;
If the Magnehelic gauge is connected with room, open the particular room door.
When the room door is in open condition the gauge needle shall comes down to zero automatically, if not adjust it to zero by means of adjusting the screw provided in front side of the gauge.
If the Magnehelic gauge is connected to the AHU, stop the equipment and wait for 5 minutes till the running blower stops completely.
When the blower stops completely the Magnehelic gauge needle comes down to zero automatically, if not adjust it to zero by means of adjusting the screw provided in front side of the gauge and Officer/Executive put a seal with sign./date shall be affixed after zero setting & preventive maintenance.
Incase Zero setting of Magnehelic gauge is unachievable then perform the investigation as per SOP, check differential pressure of the area / machine with another same to same gauge and ensure that differential pressure is maintained.
After replacing to other gauge if the zero setting is achievable then replace the faulty gauge with new Magnehelic gauge duly calibrated.
Before replacing any faulty instrument from the equipment or room premises inform to the concern department officer/executive and take necessary approval prior to replacing the instrument.
Update the instrument addition/discard record as per SOP.
Zero setting of Magnehelic Gauge shall be checked once in a month and record the parameters as per Annexure-I.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/zero-setting-of-magnehelic-gauge/
REFERENCES:
Not Applicable
ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
| Annexure-I | Zero setting of magnehelic gauge record |
ENCLOSURES: SOP Training Record
DISTRIBUTION:
- Controlled Copy No. 01 Quality Assurance Head
- Controlled Copy No. 02 Engineering Head
- Master Copy Quality Assurance Department
ABBREVIATIONS:
| SOP | : | Standard Operating Procedure |
| QA | : | Quality Assurance |
| AHU | : | Air Handling Unit |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
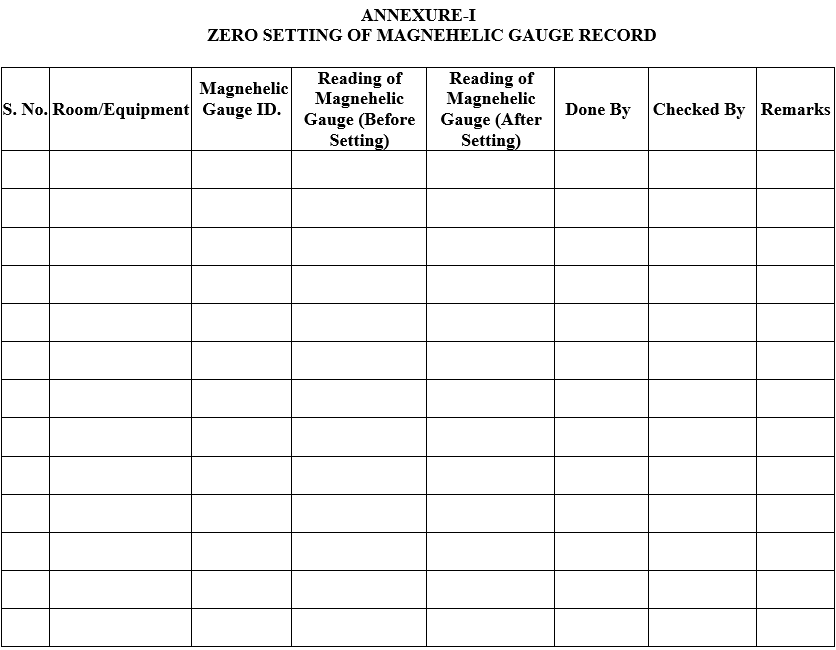
ANNEXURE-I
ZERO SETTING OF MAGNEHELIC GAUGE RECORD
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/zero-setting-of-magnehelic-gauge/