- OBJECTIVE:
- To lay down a procedure for receipt, storage and handling of microbial culture media.
- SCOPE:
- This SOP is applicable to the procedure for receipt, storage and handling of microbial culture media at {Company Name} {Location}.
- RESPONSIBILITY:
- Officer/Executive/Designee (QC) Micro. – Shall be responsible to follow the procedure as per SOP.
- Head/Designee Quality Control – Shall be responsible for ensuring compliance as per SOP.
- ACCOUNTABILITY:
- QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- Media refers to culture media, which is a specially prepared substance that provides nutrients and an ideal environment for microorganisms (like bacteria and fungi) to grow.
- Media procurement:Each dehydrated media and pre-sterilized media shall be procured only from approved manufacturer.
- Note: A change of manufacturer should require approval through Change Management. Manufacturer of dehydrated media do not require audit for approval, manufacturer of pre-sterilized media should be audited as part of the approval process.
- Receipt and storage of De-hydrated Media:
- Receive the MRR sent by stores department.
- Check the details of media mentioned in MRR against the media received and check for the Quantity received and proper packing.
- If the above mentioned details are found satisfactory, initiate move order to shift the material to microbiology laboratory.
- Upon the receipt of each media lot perform the visual inspection by verifying Code No, Batch No. / Lot No., Exp. date and storage condition against manufacturer COA.
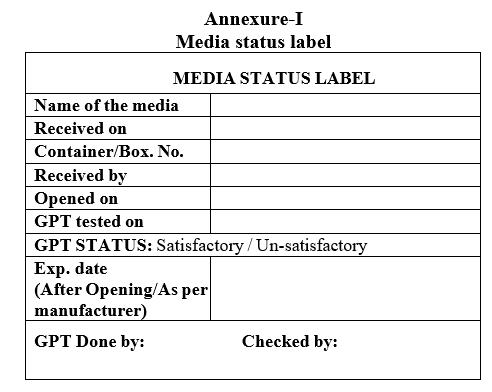
- Once the visual inspections are found satisfactory each container shall be sequentially numbered 1 of n, 2 of n, 3 of n etc., with media status label as per Format-I.
- Each receipt of dehydrated media shall be quarantined in a designated media storage area as per manufacturer storage condition mentioned, which has been mentioned on the container label.
- The first container (1 of n) of each lot shall be subjected to the physical verification by verifying the following appearance of received media and prepared media against manufacturer COA.
- Uniform colour
- Absents of lump formation
- Solubility (By dissolving required quantity in purified water and heat or boil if required).
- If any above parameter is not satisfied, the media shall be rejected.
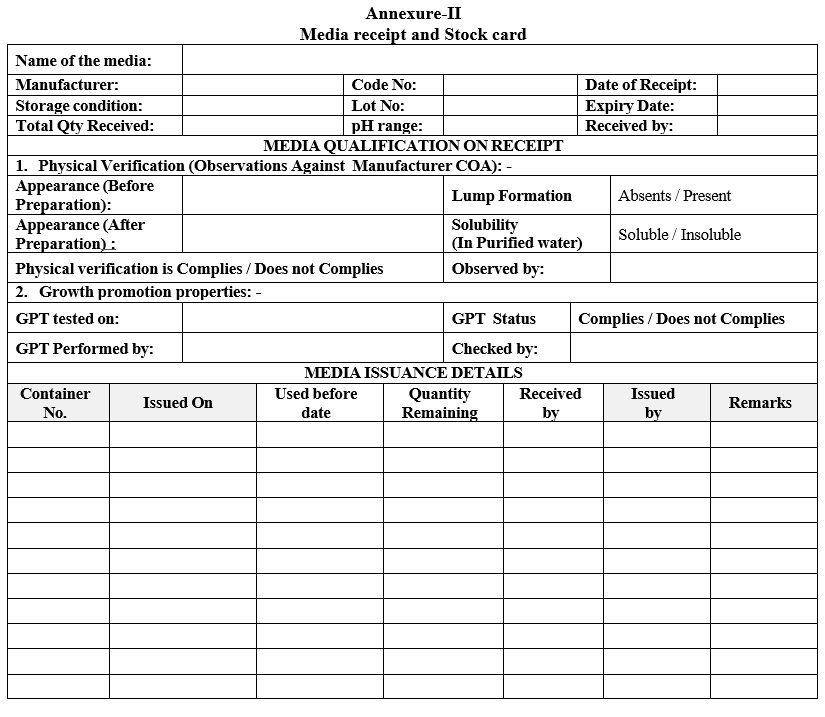
- Enter the observations in “Media receipt and Stock card” given in Format-II.
Note: Attach the manufacturer COA with Media receipt and stock card.
- Use opened containers of media within 24 months from the date of opening or manufacturer’s expiry date whichever is earlier.
- The Media storage area shall be monitored for temperature twice a day.
- Incase of any discrepancy, intimate to section In-charge, Head-Quality Control for corrective action.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/receipt-storage-and-handling-of-microbiological-culture-media/
- Receipt of Ready to use media:
- Upon receipt verify the manufacturers label on the carton boxes / media container boxes for material code no, Lot no, Mfg. Date, Exp. date, storage condition and if any other information.
- Verify details with manufacturer’s COA along with the material.
- SCDA media plates were subjected for gamma irradiation, indicator label shall be available on the pack/pouch containing SCDA plates.
- The colour of the indicator should be in orange to dark orange colour. If manufacturer is using any other specified indicator shall also be considered.
- Note: If the gamma irradiation indicator label is not present on the pack / pouch containing SCDA plates and the same lot / pouch /Box shall not be used for analysis. If label is not present the pouch /pack shall be discarded and informed to In-charge Microbiology Section or designee. If moisture observed in plates don’t use the plates for monitoring and handle the media plates as per manufacturer’s recommendation.
- Verify the media plates/broth boxes for any physical damage.
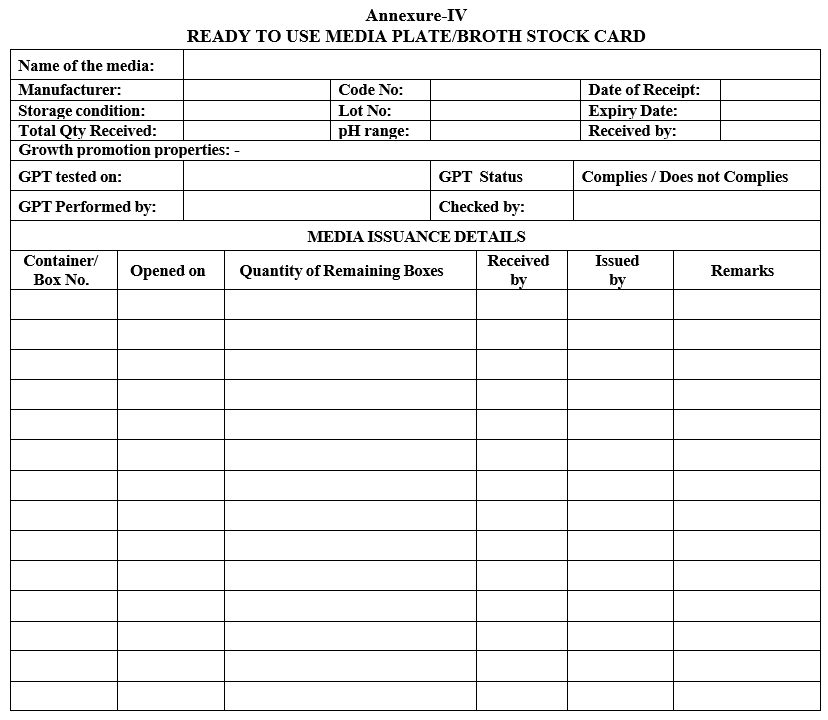
- Enter the details of receipt and make of the media in “Ready to use Media plate/Broth stock card” as per the Format-IV.
- Media container boxes shall be segregated as per the lot no. for appropriate labeling.
- All the media container boxes shall be labeled as per the following details.Each media container box shall be labeled sequentially 1 of n, 2 of n, 3 of n etc.
- Label the media container boxes as per current version of media container “Media Status Label” as per Format-I.
- The expiry date shall be given as per manufacturer recommendations.
- Transfer the Media container boxes to a designated area for storage.
- Storage of Ready to use media plates/Broth:
- Ready to use media plates/Broth shall be stored below 25°C, unless otherwise specified.
- Follow the manufacturer’s instructions where ever applicable.
- Handling of Media’s:
- Dehydrated Media:
- Perform the growth promotion test for each lot of dehydrated media with the first container 1 of n as per GTP.
- If the results meet the acceptance criteria, transfer all media from quarantine area to media under usage area and issue the dehydrated media for routine usage of Microbiology analysis if not reject the entire consignment.
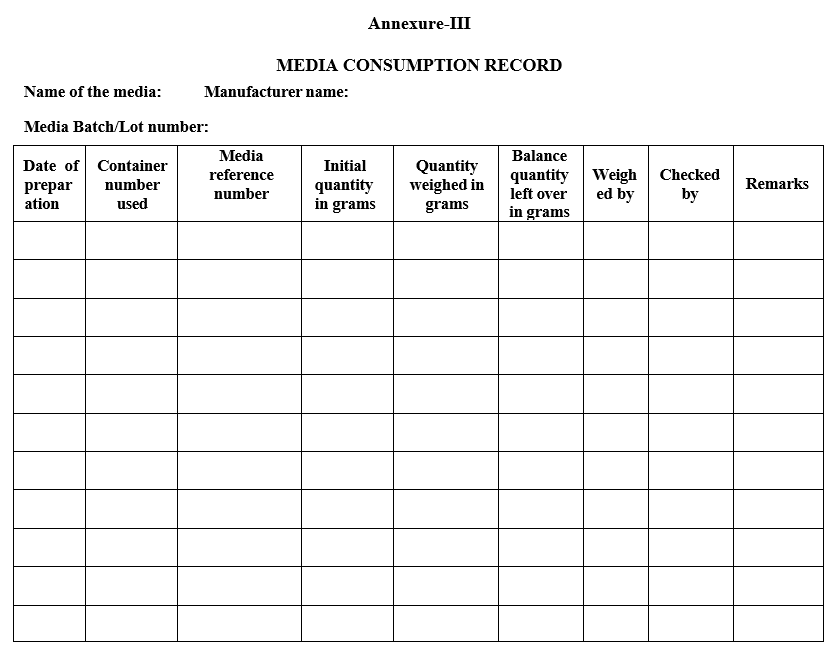
- Make an entry in Media consumption record while taking it for preparation (Format-III).
- Always follow the first in first out (FIFO) concept for the consumption of dehydrated media.
- Ready to use media:
- Enter the details in” Ready to use Media plate/Broth stock card” as per the Format-IV. pH shall be checked for each received media. \
- Upon receipt of the mediaGrowth promotion test shall be performed as per current version of GTP.
- Sufficient sample plates/Broth shall be taken from the media plate box / pack number 1 of n to perform the tests.
- Maintain a negative control along with the growth promotion test of the specified medium.
- Ready to use media plate lot details, pH and growth promotion test results shall be entered in Format.
- pH value shall meet the manufacturers recommendation or as specified.
- Usage of the Ready to use media plates:
- Prior to use, check for the triple wrap condition, droplets and any other contamination of the media pack.
- If any physical parameters are observed like plate breakage, agar cracks, agar dryness shall be informed to In-charge microbiology section.
- If the gamma irradiation indicator label is not present on the pack / pouch containing SCDA plates and the same lot / pouch /Box shall not be used for analysis.
- Whenever the growth promotion test is carried out along with the samples, ensure the growth promotion status prior to releasing the samples. If growth promotion test fails, invalidate the tests.
- Ensure the target fill volume of the agar medium in 90 mm plates shall be 18 ml – 35ml.
- If any contamination observed in the pack of agar media/individual broth media inform to In-charge microbiology section and discard the pack/ individual broth media.
- The PNC shall be raised and followed by intimation shall be given to vendor for further investigation.
- If more than 5 plates/Broth are required to be withdrawn from each received batch due to contamination the entire lot shall be rejected.
- The PNC shall be raised and followed by intimation shall be given to vendor for further investigation. The investigation shall be extended to user end.
- Until unless the investigation is completed don’t use the lot for testing.
- Reconciliation shall be done for ready to use media plates.
- Maintain a negative control for each received lot media.
- In case, ready to use media plates are not available, media plates shall be prepared in-house.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/receipt-storage-and-handling-of-microbiological-culture-media/
- ANNEXURES:
| Annexure No. | Title of annexure |
| Annexure-I | Media status label |
| Annexure-II | Media receipt and stock card |
| Annexure-III | Media Consumption Record |
| Annexure-IV | Ready to use media plate/broth stock card |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
Master Copy : Quality Assurance Department
Controlled Copy No. 01 : Head Quality Assurance
Controlled Copy No. 02 : Head QC (Micro.)
- ABBREVIATIONS:
| No. | : | Number |
| GPT | : | Growth promotion Test |
| MRR | : | Material Received Receipt |
| COA | : | Certificate of analysis |
| PNC | : | Process Non-Conformance |
| FIFO | : | First In First Out |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
Media status label
Annexure-II
Media receipt and Stock card
Annexure-III
MEDIA CONSUMPTION RECORD
Annexure-IV
READY TO USE MEDIA PLATE/BROTH STOCK CARD
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/receipt-storage-and-handling-of-microbiological-culture-media/
