- PROCEDURE FOR THE EQUIPMENT MONITORING BY SWAB AND RINSE BIOLOAD METHOD:
- Equipment Bio load:
- By Swab Method:
- Sterilize the non-sterile swab stick in 20 mL of normal saline with 0.5% Soya lecithin with 1% Polysorbate 80 in a test tube plugged with non-absorbent cotton/plastic cap and sterilize in an Autoclave as per validated cycle.
- Pre sterilized readymade swabs can also be used.
- Note: Before transferring to sampling location add aseptically 20 mL of normal saline with 0.5% Soya lecithin with 1% Polysorbate 80, incase of ready prepared swab stick are used.
- Carry the sterilized swabs in a stainless steel box, previously wiped with 70% v/v IPA to the respective area.
- Refer respective cleaning validation protocol for swab sampling locations.
- Press the swab with a rolling motion against the side of the glass tube to remove excess saline, Swab an area of approximately 25cm2 (5×5 cm) of the equipment, first with horizontal strokes followed by vertical strokes as per Figure-1.

- The swab is mixed in the saline by swirling vigorously. Label the tube with the equipment name/ID, designated location and date. Transfer the swabs samples to the microbiology department.
Note: In case any delay in analysis, store the swab samples at 2-8°C. The time elapsing between collection and examination should not exceed 72 hours. If stored at 2-8°C, allow the samples to attain the ambient temperature before analysis.
- After thorough mixing of the swab samples, filter 10 ml of saline each through 0.45 µm sterile membrane filters and rinse with 100 ml of 0.1 % sterile peptone water and place in SCDA.
- Incubate the SCDA plates at 20-25°C for 72 Hrs. followed by 30-35°C for further 48 Hrs. After completion of incubation count the colonies by using colony counter and record the results in Format-II.
- Number of colonies observed on a membrane X 2.
Example: if two colonies observed on the membrane X 2 = 4 cfu/Swab Here 10 mL used for Total Viable Count another 10 mL used for Pathogen testing.
- Transfer the remaining 10 mL sample to 90 mL of previously sterilized soya bean casein digest broth with 0.5% Soya lecithin with 1% Polysorbate 80 and incubated at 30-35°C for 18-24 hours.
- Perform test for specified organisms listed below as per GTP.
- Frequency: After every ‘type C ’cleaning/Whenever received TRF.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/equipment-monitoring-by-swab-and-rinse-bioload-method/
- Acceptance criteria:
| S. No. | Test Parameter | Limits |
| 1. | TVC (TBC+TFC) | 50 cfu/swab |
| 2. | Escherichia coli | Absent/swab |
| 3. | Salmonella spp | Absent/swab |
| 4. | Staphylococcus aureus | Absent/swab |
| 5. | Pseudomonas aeruginosa | Absent/swab |
- Rinse Bio load:
- After completion of final cleaning rinse the required equipment with specified quantity of water and collect about 100mL of this sample in a sterilized bottle. Label the bottle with the equipment name/ID, designated location and date. Transfer the bottles to the microbiology department.
Note: In case any delay in analysis, store the samples at 2-8°C. The time elapsing between collection and examination should not exceed 72 hours. If stored at 2-8°C, allow the samples to attain the ambient temperature before analysis.
- Take 10ml of this sample and filter through 0.45mm sterile membrane filters followed by 100 ml of 0.1 % peptone water place in SCDA plate.
- Incubate the SCDA plates at 20-25°C for 72 Hrs. followed by 30-35°C for further 48 Hrs. Count the number of colonies after completion of incubation.
- Count the number of colonies observed on filters / Sample volume and report as cfu/mL in Format-II.
- Take the another 10mL of the sample and transfer to 90mL of previously sterilized soya bean casein digest broth with 0.5% Soya lecithin with 1% Polysorbate 80 and incubated at 30-35°C for 18-24 hours.
- Perform test for specified organisms listed below as per GTP.
- Acceptance criteria:
| S. No. | Test Parameter | Limits |
| 1. | TVC (TBC+TFC) | 25cfu/mL |
| 2. | Escherichia coli | Absent/10mL |
| 3. | Salmonella spp | Absent/10mL |
| 4. | Staphylococcus aureus | Absent/10mL |
| 5. | Pseudomonas aeruginosa | Absent/10mL |
- Negative control:
- Negative control shall be perform by filtering with Sterile swab containing with 20 mL sterile saline solution, after filtration rinse with 100ml of 0.1% peptone water.
- Acceptance criteria: Negative control should not show any growth.
- Action plan for excursion of Acceptance criteria: If any test result exceeds the specified limits, investigation shall be carried out as per SOP and take necessary corrective action and preventive action (CAPA) shall be taken.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
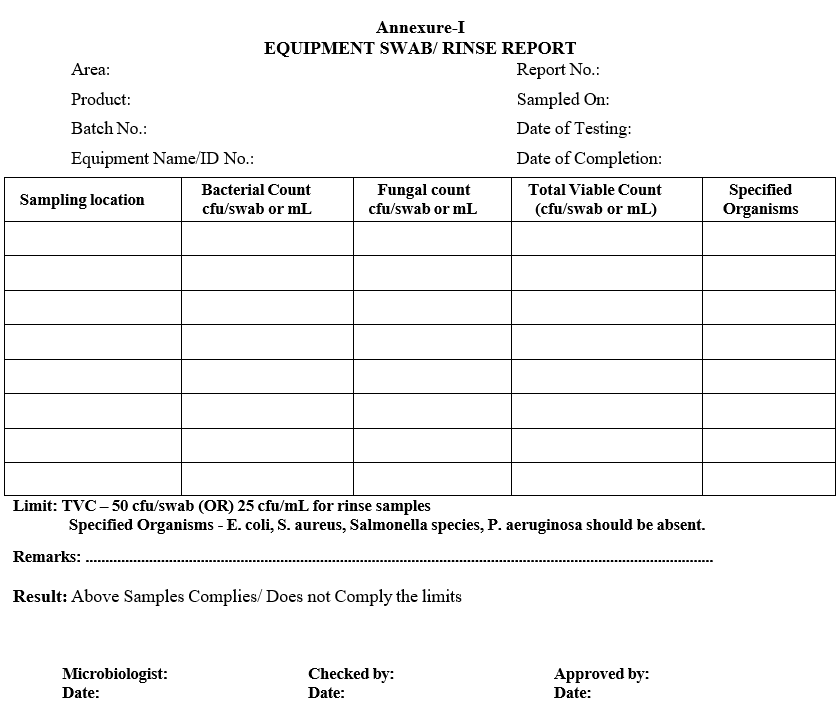
| Annexure-I | Equipment swab/ Rinse Report |
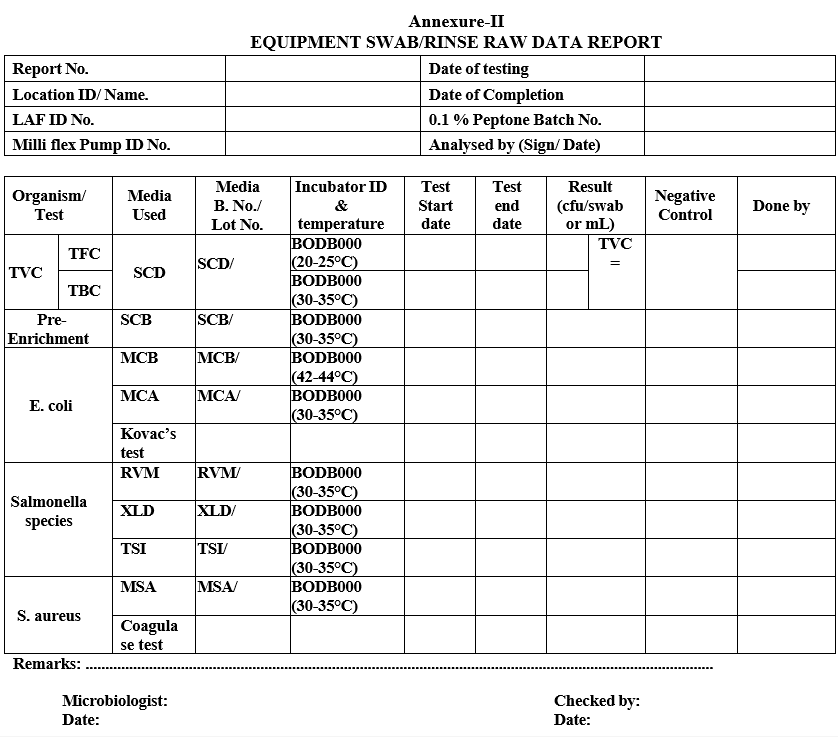
| Annexure-II | Equipment swab/rinse raw data report |
- ABBREVIATIONS:
| No. | : | Number |
| SCD | : | Soya bean Casein Digest Agar |
| SCB | : | Soya bean casein digest broth |
| cfu | : | Colony Forming Units |
| GTP | : | General test procedure |
| TRF | : | Test Request form |
| IPA | : | Isopropyl Alcohol |
| v/v | : | Volume /volume |
| TFC | : | Total Fungal Count |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/equipment-monitoring-by-swab-and-rinse-bioload-method/
Annexure-I
EQUIPMENT SWAB/ RINSE REPORT
Annexure-II
EQUIPMENT SWAB/RINSE RAW DATA REPORT